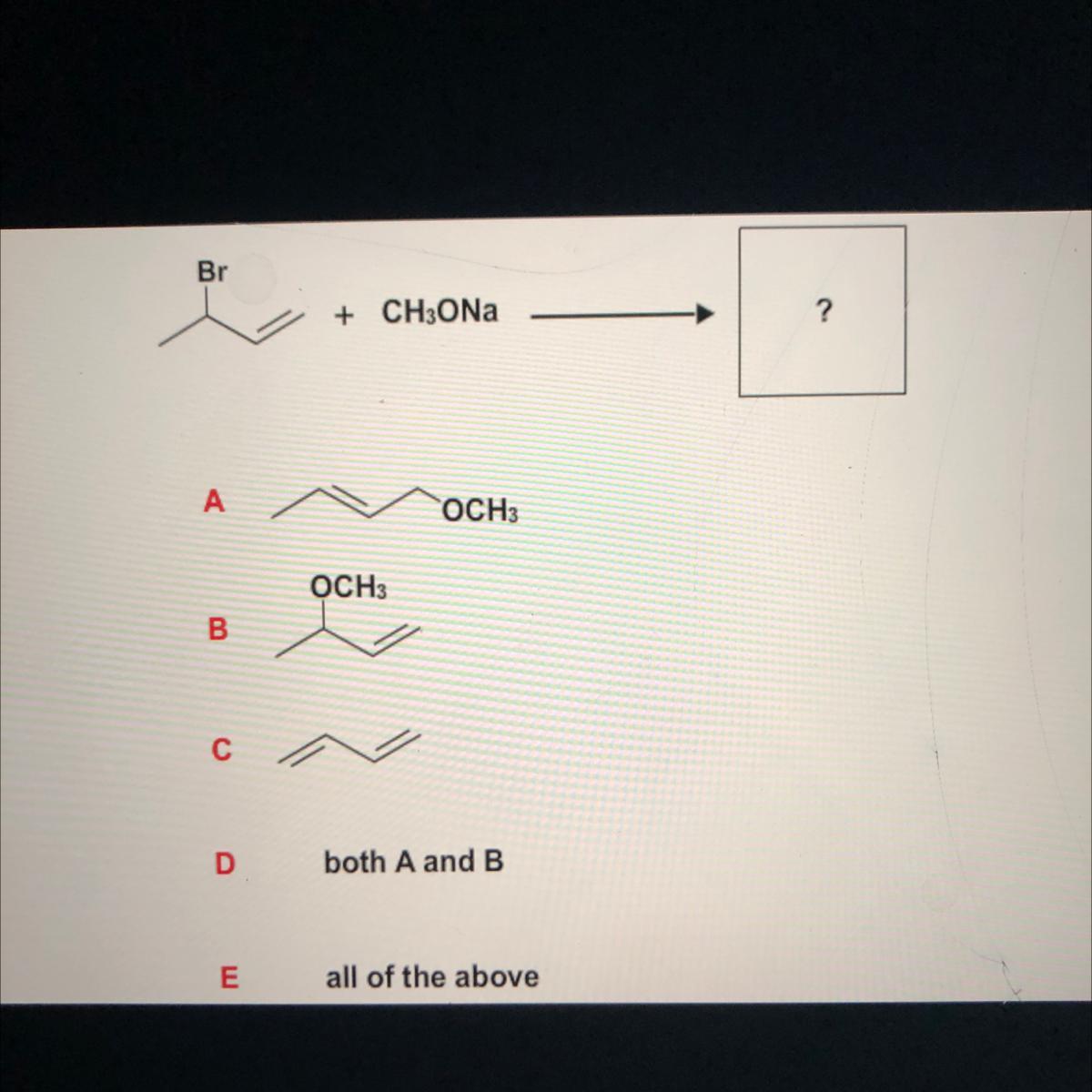
Answers
Answer:
B
Explanation:
Related Questions
Which phrase describes the element carbon-14?
Answers
We know that carbon -14 is a radioactive isotope of carbon
It is used in carbon dating to know the age of different forms of dead organism including plants...
So it is used for estimating the age of sedimentry rocks which contains dead debris of plants and animals,
it can be produced from N14 by radioactive reaction of N-14 with neutron.
It is found in organisms
Answer:
Found in organisms remains.
Explanation:
Hope this helped
Concentration data is commonly monitored during a reaction to determine the order with respect to a reactant. Consider the types of observations listed, and determine which order is likely for that reactant. Assume all other factors are held constant.
1. An increase in the concentration of the reactant in solution causes the reaction rate to increase exponentially.
a. first order
b. second order
c. zero order
2. The reaction rate increases in direct proportion to the concentration of the reactant in solution.
a. first order
b. second order
c. zero order
3. The reaction rate is constant regardless of the amount of reactant in solution.
a. first order
b. second order
c. zero order
Answers
Answer:
1) first order
2) second order
3) zero order
Explanation:
The curve of a first order reaction shows it to be exponential. In fact for a first order reaction, the concentration at a time t is an exponential function;
[A]t= [Ao] e^-kt
Where
[A]t = concentration at time =t
[Ao]= initial concentration
k= rate constant
t= time
For a second order reaction, the rate of reaction is directly proportional to the concentration of reactants.
For zero order reactions, rate of reaction is independent of concentration hence rate = k(rate constant)
The concentration data has been required for the determination of the rate of reaction. Based on the concentration of reactant and product, the rate has been determined.
1. For the first-order reaction, there has been an exponential increase in the rate of the reaction with the increase in the reactant concentration. The rate has been dependent on the concentration of the reactant.
Thus the correct option is A.
2. Irrespective of the first-order kinetic, in the second-order reaction, the rate of reaction has been directly proportional to the concentration of the reactant in the solution.
Thus option B is correct.
3. The zero-order reaction has been independent of the concentration of the reactant. The rate of reaction has been constant with an increase in the reactant concentration.
Thus option C is correct.
For more information about the rate of reaction, refer to the link:
https://brainly.com/question/8592296
WHOEVER ANSWERS THIS GETS A SHOUTOUT ON INSTA LIKE I DONT EVEN CARE HELP
The heater used in a 4.33 m x 3.43 m x 3.03 m dorm room uses the combustion of natural gas (primarily methane gas) to produce the heat required to increase the temperature of the air in the dorm room. Assuming that all of the energy produced in the reaction goes towards heating only the air in the dorm room, calculate the mass of methane required to increase the temperature of the air by 7.35 °C. Assume that the specific heat of air is 30.0 J/K-mol and that 1.00 mol of air occupies 22.4 L at all temperatures. Enthalpy of formation values can be found in this table. Assume gaseous water is produced in the combustion of methane.
Answers
Answer:
The answer is 7.89
Explanation:
Mass of methane required to increase the temperature of the air in the room by 7.35 °C is 7.95 g
The volume of air in the room is first calculated:
Volume of air in the room = 4.33 m x 3.43 m x 3.03 = 45.00 m³
1 m³ = 1000 L
45.00 m³ = 45.00 m³ * 1000 L/m³
Volume of air in L = 45000 L
Number of moles of air in 45000 L of air is then determined:
1.00 moles of air occupies 22.4 L
number of moles of air in 45000 L = 45000 L * 1 mole / 22.4 L
number of moles of air = 2008.93 moles of air
Energy that is needed to heat the room by 7.35 °C is then calculated:
Quantity of energy needed = Specific heat capacity * number of moles * temperature increase
Specific heat capacity of air = 30.0 J/K/mole
Quantity of energy needed = 30.0 * 2008.93 * 7.35
Quantity of energy needed = 442969.065 J = 443.00 kJ
The amount of methane required to produce that amount of energy is then calculated:
Equation of combustion of methane : CH₄ + 2 O₂ ---> CO₂ + 2 H₂O
Enthalpy of combustion of methane = −890.3 kJ/mole
Number of moles of methane required = 443.00 kJ / 890.8 kJ/mole = 0.497 moles
Mass of 1 mole of methane = 16.0 g
mass of 0.497 moles of methane = 16.0 * 0.497 = 7.95 g
Therefore, mass of methane required to increase the temperature of the air in the room by 7.35 °C is 7.95 g
Learn more at: https: brainly.com/question/4213585
What is the density of a block of gold that occupies 1000 ml and has a mass of 3.5 kg? Show your work
Answers
Answer:
Density of block of gold is 3.5 g/cm³.
Explanation:
Given data:
Volume of block = 1000 mL
Mass of block = 3.5 kg (3.5×1000 = 3500 g)
Density of block = ?
Solution:
Density of substance is calculated by dividing the mass of substance over its volume.
Formula:
d = m/v
d = 3500 g/ 1000 mL
d = 3.5 g/mL
or
d = 3.5 g/cm³ (1ml = 1cm³)
If the visible light spectrum is from 400 to 700 nm, would light with an energy of 2.79 x 10^-19 J be visible with the naked eye? What is the wavelength of this light?
Answers
Answer:
713 nm. It is not visible with the naked eye.
Explanation:
Step 1: Given data
Energy of light (E): 2.79 × 10⁻¹⁹ JPlanck's constant (h): 6.63 × 10⁻³⁴ J.sSpeed of light (c): 3.00 × 10⁸ m/sWavelength (λ): ?Step 2: Calculate the wavelength of the light
We will use the Planck-Einstein equation.
E = h × c / λ
λ = h × c / E
λ = 6.63 × 10⁻³⁴ J.s × 3.00 × 10⁸ m/s / 2.79 × 10⁻¹⁹ J
λ = 7.13 × 10⁻⁷ m
Step 3: Convert "λ" to nm
We will use the relationship 1 m = 10⁹ nm.
7.13 × 10⁻⁷ m × (10⁹ nm/1 m) = 713 nm
This light is not in the 400-700 nm interval so it is not visible with the naked eye.
SpongeBob noticed that his favorite pants were not as clean as they used to be and wonders how can he get them clean again? His friend Sandy told him that he should try using Clean-O detergent, a new brand of laundry soap she found at Sail-Mart. SpongeBob made sure to wash one pair of pants in plain water and another pair in water with the Clean-O detergent. After washing both pairs of pants a total of three times, the pants washed in the Clean-O detergent did not appear to be any cleaner than the pants washed in plain water.
1)What is SpongeBob’s question?
2)What is SpongeBob’s claim?
3)What is the independent variable?
4)What is the dependent variable?
Answers
Answer:
1) SpongeBob’s question is that "what would be the appropriate way to wash his favorite pants as they don't seem as clean as earlier"?
2) SpongeBob’s claim that Clean-O detergent is unable to wash the pants more cleaner as compared to plain water.
3) Independent variable in the given example is the quantity of Clean-O detergent and plain water as it can be changed or manipulated.
4) Dependent variable in the given example is the cleanliness of pants as it will be affected by the quantity of Clean-O detergent and plain water which is an independent variable.
Which of these is the term for the rate of flow of energy in a circuit?
velocity
electricity
resistance
current
Answers
4. CHALLENGE Suppose you had a mixture of sand and small,
hollow beads. How might you separate the mixture?
Answers
I'm not sure if this is the answer but maybe oil.
is carried out in a flow reactor where pure A is fed at a concentration of 4.0 mol/dm3. If the equilibrium conversion is found to be 60%, (a) What is the equilibrium constant, KC if the reaction is a gas phase reaction? (Ans.: Gas: KC = 0.328 dm3/mol)
Answers
Answer:
0.328 mol/dm³
Explanation:
We have
I started this calculation from Rate's law.
Remember equilibrium constant has been given to be 60%
Our interest is Kc, that is the equilibrium constant.
Ca = 4(1-0.6)/1+(-0.5*0.6)
= 4-2.4/1-0.3
= 1.6/0.7
= 2.2857
Cb = 4x0.6/2(1+(-0.5*0.6))
= 2.4/2(0.7)
= 2.4/1.4
= 1.7143
Kc = Cb/Ca²
= 1.7143/2.2857²
= 1.7143/5.2244
= 0.328 mol/dm³
I have added an attachment showing earlier stages to the final answer
what are the strengths in the bonds of potassium bromide
Answers
Answer: Potassium Bromide (KBr) The Ionic bond formed between Potassium and Bromine is created through the transfer of electrons from Potassium (metal) to Bromine (nonmetal).
Explanation: this type of structure departs strongly from that expected for ionic bonding and ... whose roots go back to Max Planck's explanation in 1900 of the properties of ... types of interactions between elementary particles (the strong force, the weak force, ...
Complete the reactions of Sn(II) and Sn(IV), and be sure that the reactions are balanced. Do not include the phases (liquid, aqueous, etc.). If no reaction occurs, leave the products side of the equation
completed reaction: SnBr2+PbBr4⟶
completed reaction: SnBr4+PbBr2⟶
Select the statements that are true about the reactions.
A. PbBr4 is more stable than PbBr2.
B. The inert‑pair effect renders Sn(II) as the more stable oxidation state of tin.
C. Sn(IV) is the most stable oxidation state of tin.
D. The inert‑pair effect renders Pb(II) as the more stable oxidation state of lead.
Answers
Answer:
The Inert Pair effect renders Pb(II) as the more stable oxidation state of lead
Explanation:
SnBr4 + PbBr2 ---> SnBr2 + PbBr4
SnBr2 + PbBr4 ---->
The Inert pair effect is mostly observed between group 15-17 in the periodic table. It leads to stability of the lower oxidation state of an element.
The reason for the Inert pair effect is that the s electrons become Inert due to poor shielding of the d and f-electrons. The Inert pair effect is a tendency of the s electrons not to participate in bonding (remain an Inert pair).
Owing to the Inert pair effect, Pb II is more stable than Pb IV
What is the mass in grams of 5.9 mol c8 h18
Answers
Answer:
mass = density × volume = 0.67 × 1.00 = 0.67 kg = 670 g. The molar mass of octane, C8H18, is 8 × 12.01 (C) + 18 × 1.008 (H) = 114.224.
Explanation:
The data table shows the number of pumpkin seeds that germinate at different temperatures. What is the median number of seeds that germinate at 24C?
A. 80.5 B. 82 C. 78.5 D. 80
Answers
Answer:
Explanation:
80
Name the following ionic compound: Ba(OH)2*2H2O
Answers
Metals typically lose electrons to complete their octet in a reaction with non-metals, whereas non-metals typically acquire electrons to complete their octet. Ionic compounds are typically formed via reactions between metals and nonmetals. The given compound barium hydroxide is ionic.
Ions with the opposite charge are carefully packed together to form crystalline solids. Ionic compounds typically result from reactions between metals and non-metals. The electrostatic interaction between the positive and negative ions holds ionic solids together.
Baryta, commonly known as barium hydroxide, has the chemical formula Ba(OH)₂. It is an odorless, clear-white powder. It has a toxic disposition. It is ionic in nature, with two hydroxide ions per molecule of barium hydroxide (Ba(OH)₂ in an aqueous solution an example.
To know more about ionic compound, visit;
https://brainly.com/question/13058663
#SPJ6
I need help with 5 and 6
Answers
Finally, someone gave a clear photo.
The lewis dot notation for two atoms is shown. What is represented by this notation? K loses one portion to CI, K gains one portion from CI, K loses one electron to CI, K gains one electron from CI
Answers
Answer:
K loses one electron to CI
Explanation:
The lewis electron dot notation shows only the chemical symbol of the element surrounded by dots to represent the valence electrons.
We have atom of K with one valence electrons
Cl with 7 valence electrons
For an electrostatic attraction to occur, both particles must be charged. To do this, one of the species must lose an electron, and the other gains it.
This will make both species attain a stable octet;
Hence, K will lose 1 electron and Cl will gain the electrons.
Answer:
C: K loses one electron to CI
Explanation:
I took the test and got it correct!!
How does ionic bond forms?
Answers
Answer:
Ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
Explanation: i took the same test
Answer:
it forms where a metal element and a non metal element react together to form a now compound
Write both the complete electron-configuration notation and the noble-gas notation for a barium atom.
Answers
Answer:
Explanation:
Noble gas notation: [Xe] 6s2
Complete Electron Configuration: 1s22s22p63s23p63d104s24p64d105s25p66s2.
(ik kinda hard to understand but i looked it up ant it works)
Answer:
Complete Electronic Configuration:
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²
Electronic Configuration in Noble Gas Notation:
[Xe] 6s²
determine the total amount of heat, in joules, required to completely vaporize a 50.0 gram sample of H20 at its boiling point at standard pressure
Answers
Answer: 1.13 x 10^5
Explanation:
Which of these is a source of pollution caused by humans?
A)
volcances
B)
pesticides
lightning strikes
D)
biological decay
Answers
Answer:
biological decay
Explanation:
Hope this helps
what is 19.0 ul in ml
Answers
uhhh
———————
What is ul?
A teaspoon of salt, NaCl has a mass of about
5.0 g. How many formula units are in a
teaspoon of salt?
Answers
Answer: The answer is 5.15x10^22
Explanation:
The formula unit present in a teaspoon of salt [tex]NaCl[/tex] having a mass of about 5.0 g is [tex]5.15 \times10^{22}[/tex] formula units.
Molar mass, also known as molecular weight, is the mass of one mole of a substance. It is calculated by summing up the atomic masses of all the atoms in a molecule. The unit of molar mass is grams per mole (g/mol).
Now, to determine the number of formula units in a teaspoon of salt (NaCl), we need to use Avogadro's number and the molar mass of NaCl.
Avogadro's number [tex](N_a)[/tex] is approximately. [tex]6.022 \times10^{23}[/tex] formula units per mole.
The molar mass of [tex]NaCl[/tex] is the sum of the atomic masses of sodium (Na) and chlorine ([tex]Cl[/tex]), which are approximately 22.99 g/mol and 35.45 g/mol, respectively.
To calculate the number of formula units in 5.0 g of [tex]NaCl[/tex], we can follow these steps:
Now, calculate the number of moles of [tex]NaCl[/tex] using its molar mass:
Moles = Mass / Molar mass
Moles = [tex]5.0 g[/tex] / [tex](22.99 g/mol + 35.45 g/mol)[/tex]
Calculate the number of formula units using Avogadro's number:
Formula units = [tex]Moles \times Avogadro's number[/tex]
Let's perform the calculation:
Molar mass of [tex]NaCl[/tex]= [tex]22.99 g/mol + 35.45 g/mol = 58.44 g/mol[/tex]
Moles of [tex]NaCl[/tex] = [tex]5.0 g[/tex] / [tex]58.44 g/mol[/tex] ≈ [tex]0.0856 mol[/tex]
Formula units = [tex]0.0856 mol \times (6.022 \times 10^{23})[/tex] formula units/mol ≈ [tex]5.15 \times10^{22}[/tex]formula units.
Therefore, there are approximately [tex]5.15 \times10^{22}[/tex] formula units in a teaspoon of salt ([tex]NaCl[/tex]) having mass [tex]5.0 g[/tex].
Learn more molar mass about here:
https://brainly.com/question/31545539
#SPJ2
The boundary between cold and warm air masses is called a ____.
A.
front
B.
storm
C.
climate
D.
flood
Answers
Given the volume of a gas at 200mL at 1.05atm pressure, calculate the volume of the same gas at 1.01atm. The temperature is held constant.
Answers
Answer:
The new pressure will be
1000 L
, rounded to one significant figure.
Explanation:
Boyle's law states that when a gas is held at a constant temperature and mass in a closed container, the volume and pressure vary inversely. The equation to use is
P
1
V
1
=
P
2
V
2
.
Given
V
1
=
200
mL
×
1
L
1000
mL
=
0.2 L
P
1
=
700 mmHg
V
2
=
100
mL
×
1
L
1000
mL
=
0.1 L
Unknown
P
2
Equation
P
1
V
1
=
P
2
V
2
Solution
Rearrange the equation to isolate
P
2
and solve.
P
2
=
P
1
V
1
V
2
P
2
=
(
700
mmHg
×
0.2
L
)
0.1
L
=
1400 L
, which must be rounded to
1000 L
because all of the measurements have only one significant figure
Explanation:
Please help
What subatomic particle is not important when calculating the atomic mass of an atom
Answers
Answer:
Electrons
Explanation:
This is because electrons barely contribute to the mass so only protons and neutrons are added.
What processes are related to metamorphism
Answers
Answer:
Metamorphism is the change of minerals or geologic texture (distinct arrangement of minerals) in pre-existing rocks (protoliths), without the protolith melting into liquid magma (a solid-state change). The change occurs primarily due to heat, pressure, and the introduction of chemically active fluids.
Explanation:
i hope this helps :)
Which is true of both the rock cycle and the water cycle?
both occur mostly in the atmosphere
both occur mostly in the geosphere
both have no beginning and no end
matter is created by both of them
Answers
A
Because both occur mostly in the atmosphere
And If both happens in the atmosphere it makes it the only answer by default
The study of the rocks is called petrology.
The correct answer to the question is option B that is both occur mostly in the geosphere.
What is a geochemical cycle?A geochemical cycle is the pathway that chemical elements take in the surface and crust of the Earth. The term geochemical tells us that geological and chemical factors are all included.
According to the question, both water and the rocks are present on the land and go to the reservoir with the help of a process called leaching. The common passage for both the cycling island.
Hence, the correct answer is option B.
For more information about the geochemical cycle, refer to the link:-
https://brainly.com/question/1255220
Which statement best demonstrates how data from a global positioning system (GPS) can be used to lessen the effects of a
wildfire? (1 point)
GPS data can be used by people to quickly evacuate an area because of a wildfire
GPS data can be used by scientists to predict weather patterns that can lead to a wildfire
GPS data can be used by firefighters to identify the boundaries of a wildfire
GPS data can be used by first responders to calculate the safest route to a wildfire
Answers
Answer: here is your answer
Explanation: You are visiting your Grandmother and notice that she is eating a balanced diet, taking vitamins, getting the proper amount of sleep and is not overweight. Despite her healthy lifestyle, she appears run down and tired. You realize that it's due to her lack of physical activity. Write a convincing letter to your grandma explaining the benefits of participating in regular physical activity.
The smallest form of matter that still retains the properties of an element
Answers
Answer:
atom
Explanation:
the atom is the smallest form.
Item 4
Which statement is one of the three parts of cell theory?
Cell organelles can be membrane-bound or not membrane-bound.
Cells make up tissues, tissues make up organs, organs make up systems, systems make up organisms.
The smallest living things are single cells, and cells are the functional units of multicellular organisms.
There are three types of cells, prokaryotic, eukaryotic plant, and eukaryotic animal.