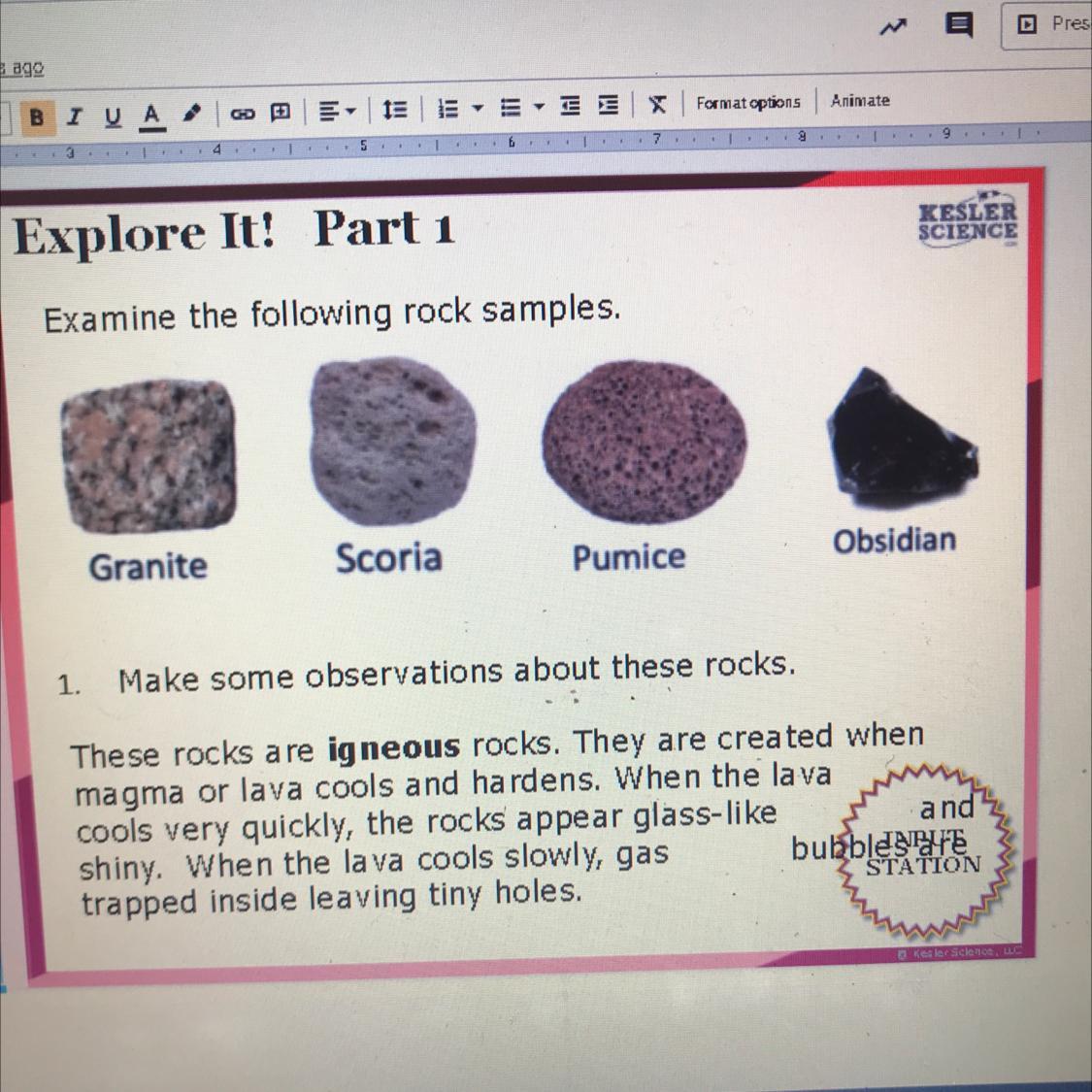
Answers
Obsidian more smooth and hard
Pumice red and bumpy
Scoria grey and rough like
Related Questions
Humans have developed ways to increase the carrying capacity of their environment. True or false
Answers
Answer:
false.nowadays humans are destroying forest,extracted more minerals,making pollution. in my view.
Air will expand about the same amount as propane with the same change in temperature over ordinary temperature ranges.
Answers
Answer:
Yes this is true
Explanation:
Answer:
i have the same question for chemistry, do you have the answer?
Explanation:
The table here provides the temperature at mount rainer in Washington state at different elevations of September 3 2014 a temperature was recorded on the same mountain at the same time the temperature was 8c at what elevation was the temperature most likely taken
Answers
Answer:
2000 m
Explanation:
From the question given above, the following data were:
Elevation (m) >>>>>> Temperature (°C)
4000 >>>>>>>>>>>>> –8
3500 >>>>>>>>>>>>> –4
3000 >>>>>>>>>>>>> 0
2500 >>>>>>>>>>>>> 4
Temperature = 8 °C
Elevation =?
A careful observation of the table shows that:
Elevation is reducing with 500 m, while the temperature is increasing with 4 °C.
Next, we shall determine number of increase in the temperature. This can be obtained as follow:
Last Temperature from the table = 4 °C
New temperature = 8 °C
Difference = 8 °C – 4 °C
Number of Increase = difference / 4 °C
Number of Increase = 4 °C / 4 °C
Number of Increase = 1
Thus, the temperature increased just ones from 4 °C to 8 °C
Since the temperature increased just ones from 4 °C to 8 °C, it means the elevation will decrease just ones i.e
Last elevation from the table = 2500 m
Decrease factor = 500 m
New elevation = 2500 – 500
New elevation = 2000 m
Thus, the temperature (i.e 8 °C) was recorded at an elevation of 2000 m
Calculate the final pH of a solution made by the addition of 10 mL of a 0.5 M NaOH solution to 500 mL of a 0.4 M HA originally at pH
Answers
This question is not complete, the complete question is;
Calculate the final pH of a solution made by the addition of 10 mL of a 0.5 M NaOH solution to 500 mL of a 0.4 M HA originally at pH = 5.0 ( pKa = 5.0)
Neglect the volume change.
Options:
a) 6.10
b) 5.09
c) 7.00
d) 5.02
Answer:
the final pH of a solution is 5.02
Option d) 5.02 is the Correct Answer
Explanation:
Given the data in the question,
Initially pKa = pH; so ratio is 1:1
thus, 0.4 M acid and base
Now, moles of NaOH = molarity × volume = 0.5 × 10 = 5 mmol = 5 × 10⁻³ mol.
Going into 500 mL ( 0.5 L ) of solution
new molarity will be;
⇒ moles / volume = 5 × 10⁻³ / 0.5 = 0.01 M
ACID reacting with BASE
original concentration of acid = 0.4 - 0.01 = 0.39 M
original concentration of base = 0.4 + 0.01 = 0.41 M
so
pH = 5 + log( base/acid)
= 5 + log ( 0.41/0.39)
= 5 + log ( 1.0512)
= 5 + 0.021
pH = 5.02
Therefore the final pH of a solution is 5.02
Option d) 5.02 is the Correct Answer
Consider a 75.0-g sample of H2O(g) at 1258C. What phase or phases are present when 215 kJ of energy is removed from this sample
Answers
Answer:
Explanation:
The first process is to draw out the heating curve to have a look at the transition possible which we've shown in the file below.
Now;
To find the energy removed to convert 75g steam → ice then comparing it with 215 kJ.
So conversion of steam at 125 °C → 100 °C
[tex]\Delta H = (75 \ g) (2.0 \ J/g^0C ) (125 - 100)^0 \ C[/tex]
[tex]\Delta H = 3750 \ J[/tex]
[tex]\Delta H = 3.75 \ kJ[/tex]
Since this is lesser than the energy given (215 kJ), we then have to find the energy removed in the next phase.
For condensation of steam
Find the energy removed to change the steam from 100° C to liquid at 100° C
[tex]\Delta H_2 = ( \dfrac{75 \ g}{18.02 \ g/mol}) (40.7 \ kJ/mol)[/tex]
[tex]\Delta H_2 =1.690* 10^ 2 \ J[/tex]
[tex]\Delta H_2 =169 \ kJ[/tex]
The total energy that is now removed from the system is:
[tex]\Delta H_1+ \Delta H_2 = (169 + 3.75) \ kJ[/tex]
[tex]\Delta H_1+ \Delta H_2 \simeq 173 \ kJ[/tex] which is still lesser than 215 kJ
To the third step; which is the conversion of water at 100° C → water at 0° C
[tex]\Delta H_3 = ( 75 \ g) (4.2 \ J/g^0 C)(100-0)^0C[/tex]
[tex]\Delta H_3 = 31500 \ J[/tex]
[tex]\Delta H_3 = 31.5 \ kJ[/tex]
Thus;
[tex]\Delta H_1+\Delta H_2+\Delta H_3 = (169 + 3.75 +31.5) \ kJ[/tex]
[tex]\Delta H_1+\Delta H_2+\Delta H_3 =204 \ kJ[/tex]
To the fourth step;
For freezing of water; we need to find the energy removed to change the water at 0° C → ice at 0° C
[tex]\Delta H_4 = (\dfrac{75 \ g}{18.02 \ g/mol})(6.02 \ kJ/mol)[/tex]
[tex]\Delta H_4 = 25.1 \ kJ[/tex]
∴
To change to the solid phase at 0° C; the total energy that is being removed from the steam is
[tex]\Delta H_1+\Delta H_2+\Delta H_3 +\Delta H_4 =(169 + 3.75 + 31.5 +25.1) \ kJ[/tex]
[tex]\Delta H_1+\Delta H_2+\Delta H_3 +\Delta H_4 =229 \ kJ[/tex]
Here, the calculated total energy is more than the given energy 215 kJ.
This implies that the water is not fully converted to ice when 215 kJ of energy removal occurs, Hence the two phases that exist are liquid and solid.
A fuel tank holds 22.3 gallons of gasoline. If the density is 0.8206 g/mL, what is the mass in kilograms of gasoline in a full tank
Answers
Answer:
[tex]m=69.3kg[/tex]
Explanation:
Hello!
In this case, since the density is computed by dividing the mass of the substance by its occupied volume (d=m/V), we first need to realize that 0.8206 g/mL is the same to 0.8206 kg/L, which means we first need to compute the volume in L:
[tex]V=22.3gal*\frac{3.78541L}{1gal}=84.415L[/tex]
Then, solving for the mass in d=m/V, we get m=d*V and therefore the mass of gasoline in that full tank turns out:
[tex]m=0.8206g/L*84.415L\\\\m=69.3kg[/tex]
Best regards!
Many children are uncomfortable around white lab coats. Specifically explain why this occurs in what a doctor or dentist could do to counteract this reaction and still wear a white lab coat
Answers
Answer:
Sanitize
Explanation:
How do we determine the central atom in a chemical bond? How information is need to determine the shape that results in the bonded atoms of the molecule?
Answers
Answer:
- the central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. If all of the atoms usually form the same number of bonds, the least electronegative atom is usually the central atom.
- molecular shape (the shape that a single molecule has) is important in determining how the molecule interacts and reacts with other molecules. Molecular shape also influences the boiling point and melting point of molecules. If all molecules were linear then life as we know it would not exist.
Uranium-238 undergoes radioactive decay according to the incomplete below. 238
92
U= 4
2 He + X.
What is the decay product represented by X?
Answers
The decay product represented by X : Thorium (Th) : ²³⁴₉₀Th
Further explanationGiven
Decay reaction
²³⁸₉₂U ⇒ ⁴₂He + X
Required
The decay product
Solution
Radioactivity is the process of unstable isotopes to stable isotopes by decay, by emitting certain particles,
alpha α particles ₂He⁴ beta β ₋₁e⁰ particles gamma particles ₀γ⁰ positron particles ₁e⁰ neutron ₀n¹In a decay reaction, the sum of the mass number and atomic number of the elements in the reactants and products must be the same
So the mass number of the element X = 238 - 4 = 234
Atomic number of the element X = 92 - 2 = 90
If we look at the periodic system, then the element with atomic number 90 is Thorium (Th)
Cells are the basic unit of life true or false
Answers
Investigate: Note the empty jars on the shelf that can be filled by using the slider. Set the amount to 2.000 moles of carbon (mol C), then press Start. Each jar holds exactly one mole of carbon. Your goal is to determine the mass in grams of two moles of carbon. Before you can find the mass, what do you need to know
Answers
Answer:
The molar mass of carbon
Explanation:
Before the mass (in grams) of two moles of carbon can be determined, the molar mass of the element would be needed.
This is because the number of mole of an element is the ratio of its mass and the molar mass. That is,
number of mole = mass/molar mass
Hence, the mass of elements can be obtained by making it the subject of the formular;
mass = number of mole x molar mass
Therefore, the molar mass of carbon would be needed before the mass of 2 moles of the element can be determined.
If silicon has a charge of +4 and oxygen has a charge of -2, what is the total charge on this structure?
Answers
Answer:
Zero with the subscripts, +2 without them.
Explanation:
Hello!
In this case, since we consider that two silicon are bonded with four oxygen atoms to form silicon oxide according to the following chemical equation:
[tex]Si^{4+}+O^{2-}\rightarrow Si_2O_4[/tex]
It is also possible to realize it can be simplified to obtain:
[tex]SiO_2[/tex]
Which means that the total charge with the subscripts is zero, and without the subscripts +2 (+4-2).
Best regards!
Which branch of chemistry would be used to make steel?
Answers
Answer:
the five major branches of chemistry are organic, inorganic, analytical, physical, and biochemistry.
1: draw the structural formula for N2 + 3H2 → 2NH3
2: draw the structural formula for 2CO2 + 3H2O → C2H5OH+ 3O2
]down below is a picture with more accurate depiction of the questions plz answer both
Answers
Answer:
Hey..! I know only 1st one answer
alkenes a. none of the above b. are relatively polar compounds c. are reasonably soluble in water d. have lower boiling points than alcohols of similar molecular weight
Answers
Answer:
have lower boiling points than alcohols of similar molecular weights.
Explanation:
Alkenes are hydrocarbons of the general molecular formula CnH2n. Alkenes are nonpolar hydrocarbons that are insoluble in water.
We must recall that alcohols are able to associate in water due to hydrogen bonding. This causes alcohols to have a relatively higher boiling point than alkenes of comparable molecular weight. Hencethe answer above.
Calculate the molarity of a solution prepared by dissolving 15.0 moles of solute in enough solvent to 50.0 liters of solution.
Answers
Answer:
Molarity of a solution = 0.3 mol/liter
Explanation:
Given:
Moles of solute = 15 moles
Amount of solution = 50 liters
Find:
Molarity of a solution
Computation:
Molarity of a solution = Moles of solute / Amount of solution
Molarity of a solution = 15 / 50
Molarity of a solution = 0.3 mol/liter
pleaseeeeeee helpppppppppp
Answers
Answer:It is number 4
Explanation:
What is the mass of two moles of the diatomic gas, Nz?
Answers
Answer:
56.04 g
Explanation:
Step 1: Given data
Moles of nitrogen (n): 2 mol
Step 2: Calculate the molar mass of nitrogen (M)
The molar mass of the molecule is the sum of the masses of the molecules that form it.
mN₂ = 2 × mN = 2 × 14.01 g/mol = 28.02 g/mol
Step 3: Calculate the mass corresponding to 2 moles of nitrogen
We will use the following expression.
m = n × M
m = 2 mol × 28.02 g/mol = 56.04 g
50 POINTS PLEASE NO FAKE ANSWERS I REALLY NEED THESE ANSWERED
1. The following reaction shows calcium chloride reacting with silver nitrate.
CaCl2 + 2AgNO3 → 2AgCl + Ca(NO3)2
How many grams of AgCl are produced from 30.0 grams of CaCl2?
(Molar mass of Ca = 40.078 g/mol, Cl = 35.453 g/mol, O = 15.999 g/mol, Ag = 107.868 g/mol, N = 14.007 g/mol)
19.4 grams
38.8 grams
58.2 grams
77.5 grams
2. The table shows the recipe and the available ingredients for making the maximum possible number of sandwiches.
Making Sandwiches
Recipe for One Sandwich Ingredients Available
2 cheese slices, 1 ham slice, 2 bread slices 12 cheese slices, 10 ham slices, 12 bread slices
If the ingredients represent reactants of a chemical reaction, which of the following represents the leftover reactant?
Two ham slices
Four ham slices
Two cheese slices
Four cheese slices
3. Read the given chemical reaction.
C2H6 + O2 → CO2 + H2O
How many moles of H2O are produced during the complete combustion of 1.4 moles of C2H6?
2.8 moles
4.2 moles
5.6 moles
7.0 moles
4. The image represents the reaction between a certain number of molecules of N2 and H2.
[IMAGE INCLUDED]
If the maximum possible amount of NH3 is formed during the reaction, what is the leftover reactant?
One molecule of N2
One molecule of H2
Two molecules of N2
Two molecules of H2
Answers
what is the atomic number of an atom woth six valence electrons?
(A) 8
(B)10
(C) 12
Answers
Answer:
8
Explanation:
The atomic mass of an atom refers to the number of protons in the nucleus of an atom. Hence, an element with 6 valence electron mean that it has 6 electrons in its outermost shell.
The atomic Number of elements with 6 valence electrons cannot necessarily be the same, however, elements with 6 valence electrons belong to group six on the periodic table.
From the options given above, :
Atomic number of 8 :
Configuration : 2, 6
Atomic number of 10 :
Configuration : 2, 8
Atomic number of 12 :
Configuration : 2, 8, 2
Hence, only the element with atomic number of 8 has 6 valence electrons in its outer most shell, hence, the answer.
Consider 55 mL of water (H2O) in a beaker and 55 mL of acetone [(CH3)2CO] in an identical beaker under identical conditions. Complete the sentences to explain the relationship between the vapor pressure of water and acetone.
Answers
Answer:
More slowly than
Larger than
Weaker
Explanation:
The vapor pressure of a solution describes how quickly a solution will turn into vapour. If we say that a solution has a high vapour pressure, we are actually saying that the solution turns into vapour more quickly.
Acetone has a higher vapour pressure than water so acetone turns to vapour quicker than water.
Vapour pressure has a lot to do with the magnitude of intermolecular forces in solution. The stronger the magnitude of intermolecular forces, the lower the vapor pressure and the more slowly the solution evapourates.
So, the hydrogen bonds in water provides stronger intermolecular forces than dispersion and dipole interaction in acetone. Hence, water vaporizes more slowly than acetone.
Name of this compound:
S3P2
Answers
Answer:
sodium phosphide
Explanation:
Under certain conditions, the substance ammonium chloride can be broken down to form ammonia and hydrogen chloride. If 29.4 grams of ammonium chloride react to form 9.4 grams of ammonia, how many grams of hydrogen chloride must simultaneously be formed
Answers
Answer: 20.0 g of hydrogen chloride must simultaneously be formed
Explanation:
The balanced chemical reaction is :
[tex]NH_4Cl\rightarrow NH_3+HCl[/tex]
According to the law of conservation of mass, mass can neither be created nor be destroyed. The mass on reactant side must be equal to the mass on product side.
Thus mass of reactants = mass of products
Given : mass of ammonium chloride = mass of reactants = 29.4 g
mass of ammonia = 9.4 g
mass of products = mass of ammonia + mass of hydrogen chloride
9.4 g +mass of hydrogen chloride = 29.4 g
mass of hydrogen chloride = 20.0 g
Please help if you know please and thanks ;)
Answers
Answer:
it takes two hydrogen atoms for every oxygen atom to produce this reaction, so the mole ratio between hydrogen and oxygen is 2:1
What would be the mass of 9.03 x 1021 molecules of hydrobromic acid?
Answers
Answer:
[tex]1.214\ g[/tex]
Explanation:
[tex]We\ know\ that,\\No.\ of\ molecules\ of\ Hydrobromic\ acid=9.03*10^{21} HBr\ molecules\\Now,\\We\ know\ that\ Hydrobromic\ acid\ is\ constituted\ by\ 1\ Hydrogen\ molecule\\ and\ 1\ Bromine\ molecule.\\Gram\ Atomic\ Mass\ of\ Bromine \approx 80\ g\\Gram\ Atomic\ Mass\ of\ Hydrogen =1\ g\\Hence,\\The\ Gram\ Molecular\ Mass\ Of\ Hydrobromic\ Acid=1*1+1*80=81\ g\\Avagadro's\ Constant=6.022*10^{23}\ particles[/tex]
[tex]Now,\\We\ know\ that,\\Mass=\frac{No.\ of\ particles}{Avagadro's\ Constant}*GMM\\Here,\\Mass\ of\ 9.03*10^{21} molecules\ of\ HBr= \frac{9.03*10^{21}}{6.022*10^{23}}*81 \approx 1.214\ g[/tex]
how does heat transfer into conduction
Answers
Explanation:
Heat is transferred by conduction when adjacent atoms vibrate against one another or electrons move from one atom to another . Conduction is the most significant meanings of heat transfer within a solid or between solid objects informal contact.
What is the molar mass for Ca(C2H3O2)2?
A. 71g B. 99g C. 158g D. 148g
Answers
40.078+(12.0107*2 + 1.00794*2 + 15.9994*2)*2
If a solution has equal numbers of hydronium
and hydroxide ions, what is its pH?
ANSWER NOWW PLS I NEED HELP
Answers
Answer:
neutral!
pH = 7
Explanation:
⁄(⁄ ⁄•⁄ω⁄•⁄ ⁄)⁄
What is the relationship between particle collisions and reaction rates?
Answers
Answer:
Collision theory states that the rate of a chemical reaction is proportional to the number of collisions between reactant molecules. The more often reactant molecules collide, the more often they react with one another, and the faster the reaction rate.
PLZZZ help asaap plz its 50 points plzzzz Calculate for the formula mass (for ionic compounds) and molecular mass (for covalent compounds):
Mg3 (AlO3)2
(NH4)2 C2O4
Al 4 ( Fe (CN)6)3
Answers
hope it helps
mark me brainliest