Help! Please:(
Given the following chemical equation, how many grams of water are created when 50.0 grams of oxygen react with excess hydrogen ?
2H2 + O2 - -> 2H20
Answers
Answer:
About 56.3 grams of H2O
Explanation:
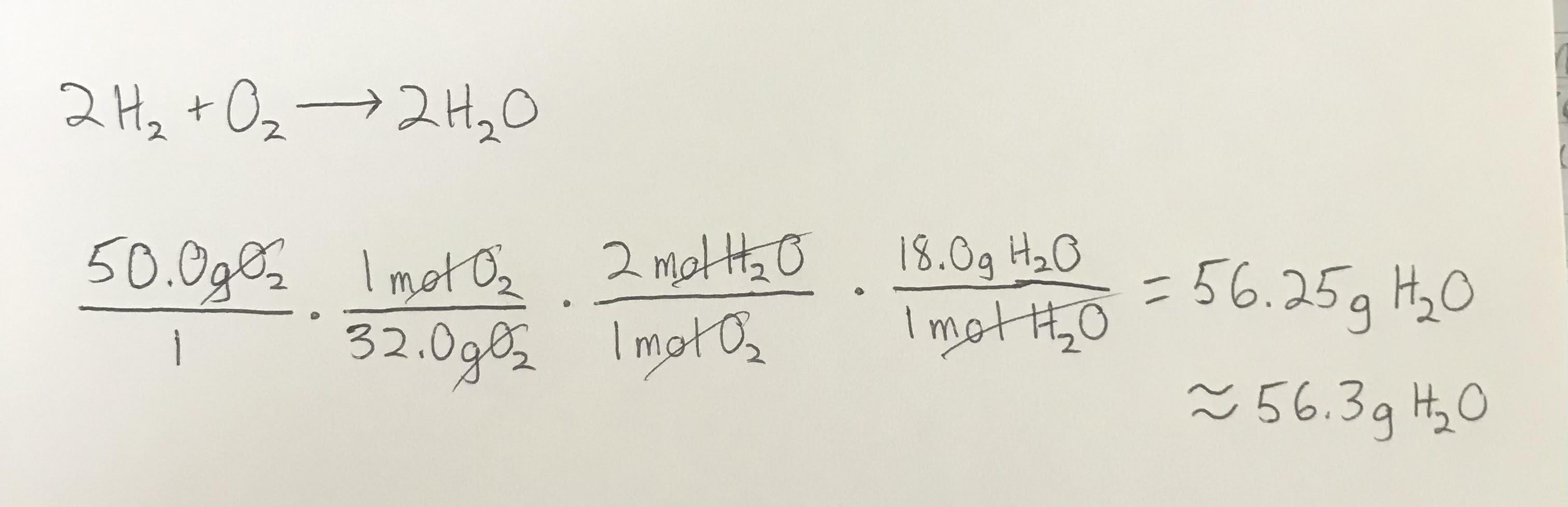
Related Questions
CAVA Chemistry 302/303B Unit 2 Lab Report
THE MYSTERY SALT
Imagine that you have a barrel of salt, but you forgot to label it. You know it must be either KNO3, or KCl.
You look at the solubility curves for KNO3 and KCl and you find that at 35 degrees Celsius, 100 g of water can dissolve about 30 g of KNO3, or about 37 g of KCl.
The solubility curves disappear. You only remember the solubility for both salts at 35 degrees Celsius in 100g of water. You know absolutely nothing else about these salts.
You have a scale, a hot plate, a thermometer, empty beakers, and plenty of water. You do NOT have any labeled KNO3 or KCl.
1. What property can you use to determine whether the barrel contains KNO3 or KCl? Hint: Name the property you could use to identify the mystery salt? (1 point)
2. Explain exactly what you would do. Another person should be able to perform your test by following your procedure. Do not bother with why you are doing these steps. Just tell me exactly what to do to perform this test. Hint: Do NOT include any discussion of your results. Save that for #3. (2 points)
3. How would your results identify the mystery salt? Hint: What exact results would indicate KNO3? What results would indicate KCl? (2 points)
Name:
Answers
1 (Property):
2 (Procedure):
3 (Results):
Answers
2 procedure
Results
what happens to the boiling point of hydrocarbon compounds when the number of carbon atom increases
A. Decreases
B. Increases
C. Remains the same
D. None of these
I NEED YOUR ANSWER RN GUYSS PLEASEE
Answers
So therefore your answer choice will increase. (B)
blank a long narrow Stepside value that forms the deepest part of the ocean
Answers
Answer:
The answer is a trench
Na2SO4
How many oxygen stems are?
Answers
Answer:
Na2SO4 means: two moles sodium (45.98 g), one mole sulfur (32.06 g), and four moles oxygen (64.00 g) combine to form one mole of sodium sulfate (142.04 g).
Explanation:
Answer:
As we can see in the formulae, Na₂SO₄
We can see that 4 is the subscript of O and that means There are 4 atoms of oxygen.
What is the formula for the compound formed by Al3+ and N3- ?
Answers
Answer:
[tex]\boxed {\boxed {\sf AlN}}[/tex]
Explanation:
The goal when forming compounds is to achieve a net charge of 0.
We are given aluminum with a charge of 3+ and nitrogen with a charge of 3-.
These charges are equal and opposite, so one of each will equal 0.
3+ (Al) + 3- (N) = 0Therefore, we do not need any subscripts. List the metal's symbol (aluminum) then the nonmetal.
AlNTh formula for the compound, which is aluminum nitride, is AlN.
Is the reaction above a replacement reaction? Explain.
Answers
Name two sources of cholesterol in the human body
Answers
Answer:
The cholesterol in your blood comes from two sources: the foods you eat and your liver. Your liver makes all the cholesterol your body needs.
Will mark BRAINLIEST. Molarity
Please no Bs answers. Only going to be reported.
If water is added to 145 mL of a 0.55 M KOH solution until the volume is 250 mL, what will the molarity of the diluted solution be?
What is the molarity of the solution that results from diluting 35.0 ml of a 9.02M solution to a new volume of 45.0 ml?
Answers
We can use the equation for dilutions that relates concentration and volume:
[tex]M_1V_1=M_2V_2[/tex]
where M is the molarity, V is the volume, and 1 and 2 refer to the initial and final states of the solution, respectively. Here, we are given the molarity of the initial solution and the volumes of the initial and final (diluted) solutions. To find the final concentration (i.e., the molarity of the diluted solution), we would be solving for M₂:
[tex]M_2=\frac{M_1V_1}{V_2} = \frac{(0.55 \text{ M})(145 \text{ mL})}{250 \text{ mL}} \\ M_2 = 0.319 \approx 0.32 \text{ M}.[/tex]
The molarity is given to two significant figures as both our M₁ and V₂ are given to two significant figures.
Note: Although our volumes are in mL instead of L, we do not need to convert them to L for the purposes of our calculation since we would be multiplying our V₁ and V₂ by a common factor that would cancel out in division. All that matters is the ratio between the two volumes, which is the same whether the volumes are in mL or in L.
---
We follow the exact same procedure in the second question as we did in the first problem: solve for M₂ given M₁ = 9.02 M, V₁ = 35.0 mL, and V₂ = 45.0 mL:
[tex]M_2=\frac{M_1V_1}{V_2} = \frac{(9.02 \text{ M})(35.0 \text{ mL})}{45.0 \text{ mL}} \\ M_2 = 7.02 \text{ M}.[/tex]
Which of the following best describes a solution?
a heterogenous compound
a homogenous compound
a homogenous mixture
O O a heterogenous mixture
Answers
Answer:
homogeneous mixture
How to make copper chloride from copper carbonate
Answers
Answer:
Describe how a sample of copper chloride crystals could be made from copper carbonate and dilute hydrochloric acid. Add excess copper carbonate to hydrochloric acid in a beaker, stirring until there is no further reaction. Filter the mixture to remove the remaining copper carbonate, then heat the remaining solution to the point of crystallisation.
Explanation: Hope this is right.
A reaction is expected to produce 28.3 moles of hydrogen gas. If the hydrogen is collected at 297 K and 1.08 atm, what is the volume? 305 L H2 639 L H2 948 L H2 1,240 L H2
Answers
Answer:
The correct answer is 639 L H₂
Explanation:
We use the ideal gas equation:
We have the following data:
n = 28.3 mol
T= 297 K
P= 1.08 atm
R= 0.082 L.atm/K.mol (gas constant)
We introduce the data in the gas equation to calculate the volume (V):
PV=nRT
⇒V =nRT/P = (28.3 mol x 0.082 L.atm/K.mol x 297 K)/(1.08 atm) = 638.2 L ≅ 639 L
Therefore, the correct option is 639 L H₂
Answer:
639 L H2
Explanation:
took the quiz
ur welcome
is lime flavor ionic or covalent
Answers
Answer:Calcium carbonate (CaCO3), essentially, is an ionic compound having the bivalent calcium and carbonate ions. But the carbonate anion is a polyatomic species. The carbon atom is bonded to all the three oxygen atoms by covalent bonds - two carbon-oxygen single bonds and one carbon-oxygen double bond.
Explanation:So it's ionic hope this helps u. Btw may i have brainlist plz.
A horse weighs 240 kg. If its running at a speed of 18 m/s, what's the linear momentum of the horse
Answers
Answer: The answer is 4320
Explanation:
I got the answer correct
Question 1
1. Na2O + H20 --->
NaOH
A. Single Replacement
B. Double Replacement
c. Decomposition
D. Synthesis
E. Combustion
Answers
Answer: D. Synthesis
Explanation:
Sodium Oxide + Water = Sodium Hydroxide
If Steve throws a football 57 meters in 3 seconds, what is the average speed of the football?
Answers
Answer:
19 m/s
Explanation:
57/3
80 ml of carbon monoxide (CO) are reacted with 40 ml of oxygen (O2). What volume of carbon dioxide (CO2) is formed? 2CO + O2 → 2CO2
a. 200 ml
b. 120 ml
c. 80 ml
d. 40 ml
Answers
The volume of carbon dioxide is formed is Option C i.e. 80 ml.
Given that,
80 ml of carbon monoxide (CO) are reacted with 40 ml of oxygen ([tex]O_2[/tex]).Based on the above information, the calculation is as follows:
Here the volume should be 80ml
Therefore we can conclude that The volume of carbon dioxide is formed is Option C i.e. 80 ml.
Learn more: brainly.com/question/16911495
Use the balanced chemical equation below to answer the following: How many moles of
oxygen are required to react along with 6 moles of butane (C4H10) according to the
following equation? Do not list units in your final answer.
2 C4H10 + 13O2 ------> 8CO2 + 10H2O
Answers
hmm, i not sure i come back later with the answer.
Which is NOT a way nitrogen can be fixed or made more useful?
a
lightning
b
Nitrogen fixing bacteria
c
denitrification
d
assimilation
Answers
Answer:
A lightning
Explanation:
A brick measures 0.018 dam by 6.5 cm by 17.3 cm. What is the volume of the brick in cubic centimeters
Answers
Answer:
volume of brick= length × breadth × height
=0.000018 ×6.5×17.3
=
What 3 things will you do to dissolve a solid (salt) faster in a liquid (water)
Answers
Answer:
There are three ways to make solids dissolve faster: Break the solute into smaller pieces. Stir the mixture. * Heat the mixture.
Explanation:
Substances can dissolve in water three ways—by dissociation, dispersion, and ionization.
How many significant digits are in this number?
742800
1.6
2.5
3.4
4.3
Answers
The mass of a single gold atom is 3.27X10^-22 grams. How many gold Adams with there be in 57.8 mg of gold.
Answers
Answer:
18 * 10^19 atoms
Explanation:
We must first convert 57.8 mg to grams.
If 1000 mg = 1g
57.8 mg = 57.8/1000 = 57.8 * 10^-3 g
Now;
If 1 gold atom has a mass of 3.27X10^-22 grams
x gold atoms have a mass of 57.8 * 10^-3 g
x = 57.8 * 10^-3 g/3.27X10^-22 g
x = 18 * 10^19 atoms
What is the pH of a 2.0 × 10-4 M HCl solution?
Answers
Answer:
pH = 3.69
Explanation:
HCl is a strong acid. It will disscociates completely into dissoluted ions such that,
[tex][H^+]=2\times 10^{-4}\ M[/tex]
The formula for pH is given by :
[tex]pH=-log[H^+]\\\\pH=-log(2\times 10^{-4})\\\\pH=3.69[/tex]
So, the pH of the solution is 3.69.
The answer to your question is 4.
Which of these indicates that a liquid has transferred thermal energy to the air?
O The liquid increases in temperature, and its particles lose kinetic energy
O The liquid decreases in temperature, and its particles gain kinetic energy
O The liquid increases in temperature, and its particles gain kinetic energy.
O The liquid decreases in temperature, and its particles lose kinetic energy
No
Answers
Answer:
The liquid decreases in temperature and its particles lose kinetic energy.
Temperature is proportional to kinetic energy, so if temperature decreases, kinetic energy decreases. Energy has been removed from the liquid, because it is in the air
if answered correctly i will give brainlest
Answers
Answer: Earthquake location
Explanation:
Answer:
Volcano chains and arcs, and earthquake locations
Need help ASAP please and thank you
Answers
need help to solve it
Answers
Answer:
Divide by the molar mass to get moles
Explanation:
What is the correct formula for sodium carbonate?
a. Na(CO3)2
b. Na,(CO3)2
C. Na2CO3
Naz(CO)2
e. NaCO3
d.
a
a
b
b
ΟΟΟΟΟ
С
С
d
d
е
e
Answers
Answer:
na2 co3
Explanation:
sodium carbonate formula
Answer:
your answer is C.
Explanation:
this is what it would be like
O
|
|
Na+ C Na+
/ \
/ \
O- O-
How many ATOMS are in 7.32 moles of sulfur
dioxide?
Answers
Using the concept of Avogadro's number, the number of atoms present is 4.4 * 10^24 atoms.
What is Avogadro's number?Professor Avogadro provided a nice way of calculating the number of atoms in a molecules by the use of the Avogardro's number. Now we know that 1 mole of a substance contains about 6.02 * 10^23 atoms, molecules ions etc.
Hence;
1 mole of sulfur dioxide contains 6.02 * 10^23 atoms
7.32 moles of sulfur dioxide contains 7.32 moles * 6.02 * 10^23 atoms/1 mole
= 4.4 * 10^24 atoms.
Learn more about Avogadro's number: https://brainly.com/question/1445383?
how many grams of iorn reacts with 71 grams of chlorine to produce 117 grams of sodium chloride
Answers
Answer:
The answer to your question is: 71 g of Chlorine
Explanation:
Na = 46 g
Cl = ?
NaCl = 117 g
Equation
2 Na + Cl₂ ⇒ 2 NaCl
46g X 117g
If we consider the law of conservation of matter, we know that the mass of the reactants equals the mass of the products, then
mass Na + mass Cl₂ = mass NaCl
46 g + X = 117
X = 117 - 46
x = 71 g of Chlorine