Draw hydrogen cyanide lewis structure
Answers
The hydrogen cyanide (HCN) lewis structure has a triple bond between the carbon (C) and nitrogen (N) atoms, with the hydrogen (H) attached to the carbon.║
Hydrogen cyanide is a polar molecule due to the unequal sharing of electrons between the C and N atoms. The C atom pulls the electrons more strongly, giving it a slight negative charge, while the H atom has a slight positive charge.
This polarity allows hydrogen cyanide to form hydrogen bonds with other polar molecules, making it a useful solvent and a building block for organic chemistry. However, hydrogen cyanide is also a highly toxic gas and can cause severe health effects if inhaled or ingested.
To know more about the lewis structure, here
brainly.com/question/20300458
#SPJ4
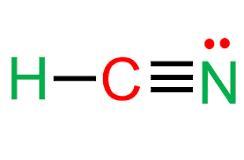
Related Questions
Which temperature change would cause a sample of an ideal gas to double in volume while the pressure is held constant?
Answers
A temperature change from 200 K to 400 K, which is a doubling of the temperature would cause a sample of an ideal gas to double in volume while the pressure is held constant. Option (2)
According to Charles's law, at constant pressure, the volume of a gas is directly proportional to its temperature (in kelvins). Mathematically, this relationship can be expressed as V₁/T₁ = V₂/T₂, where V is volume and T is temperature.
If the volume of an ideal gas is to double while the pressure is held constant, the final volume (V₂) must be twice the initial volume (V₁). Therefore, using the above equation, T₂ must be twice T₂.
Options (1), (3), and (4) do not describe a doubling of temperature, so they would not cause a doubling of volume at constant pressure.
The complete question is;
Which temperature change would cause a sample of an ideal gas to double in volume while the pressure is held constant?
(1) from 400 K to 200 K
(2) from 200 K to 400 K
(3) from 400 ⁰C to 200 ⁰C
(4) from 200 ⁰C to 400 ⁰C
Want to know more about an ideal gas visit the link which is given below;
https://brainly.com/question/14934878
#SPJ4
Chemistry help needed
Answers
Answer: If I would have to make an educated guess, it would probably be the curve that is mostly flat
Explanation:
The O-H stretch of a concentrated solution of an alcohol occurs at a lower frequency than the O-H stretch of a a dilute solution. True or False
Answers
The statement that the O-H stretch of a concentrated solution of alcohol occurs at a lower frequency than the O-H stretch of a dilute solution is False.
Does the O-H stretch of a concentrated solution of alcohol occur at a lower or higher frequency than the O-H stretch of a dilute alcohol solution?The O-H stretch of a concentrated solution of alcohol occurs at a higher frequency than the O-H stretch of a dilute solution.
This is because, in a concentrated solution, there are more intermolecular hydrogen bonding interactions between the alcohol molecules, which leads to a stronger bond and higher vibrational frequency. In a dilute solution, there are fewer intermolecular hydrogen bonding interactions, resulting in a weaker bond and lower vibrational frequency.
Learn more about O-H stretch of alcohol at: https://brainly.com/question/30425622
#SPJ1
one mole of the ionic compound, nacl, will dissolve into _ _ _ _ _ _ _ moles of particles in aqueous solution.
Answers
one mole of the ionic compound, nacl, will dissolve into 6 moles of particles in aqueous solution.
What is ionic compound?
An ionic compound is a type of chemical compound that consists of ions held together by ionic bonds. Ionic compounds are formed when positively charged ions (cations) bond with negatively charged ions (anions). The cations are formed from metals, while the anions are formed from nonmetals. Ionic compounds are usually soluble in water and are typically solid at room temperature. Examples of ionic compounds include sodium chloride (NaCl), magnesium sulfate (MgSO4), and aluminum oxide (Al2O3). Ionic compounds are found in everyday items such as table salt, baking soda, and bleach.
Therefore, one mole of the ionic compound, nacl, will dissolve into 6 moles of particles in aqueous solution.
To learn more about ionic compound
Here: https://brainly.com/question/29473901
#SPJ4
Help please will give brainliest ! How do intracellular communication and intercellular communication differ?
A. Intracellular communication involves passing molecules through gap junctions
B. Intercellular communication involves moving a molecule within a cell
C. Intercellular communication occurs between two different cells
D. Intracellular communication occurs between two different cells
Answers
Answer:
its c.
Explanation:
''intercellular communication occurs between two different cells''
Why are there significantly more thunderstorms in Florida than in California?a. The state of Florida is smaller than the state of California.b. The air in Florida is more stable than the air in California.c. The air above Florida holds less moisture than the air above California.d. The ocean currents near Florida are warmer than the ocean currents near California.
Answers
There are significantly more thunderstorms in Florida than in California D: "The ocean currents near Florida are warmer than the ocean currents near California".
The primary reason why there are significantly more thunderstorms in Florida than in California is because of the difference in ocean currents. The ocean currents near Florida, specifically the Gulf Stream, are much warmer than the currents near California. This leads to a more unstable atmosphere, which creates the ideal conditions for thunderstorm formation. In addition, the warm and moist air that moves inland from the Atlantic and the Gulf of Mexico also contributes to the formation of thunderstorms in Florida.
You can learn more about thunderstorms at
https://brainly.com/question/20164152
#SPJ4
What is the energy of ATP?
Answers
One ATP molecule hydrolyzes to provide 7.3 kcal/mol of energy.
ATP (adenosine triphosphate) is a molecule that stores and transports chemical energy within cells. The energy stored in ATP is used to power many cellular processes, including metabolism, protein synthesis, and cell division.
The energy in ATP is stored in the bonds between its three phosphate groups. When one of these bonds is broken, energy is released and ATP is converted to ADP (adenosine diphosphate). This energy can then be used to power up cellular processes.
The energy of ATP is stored in the bonds between its phosphate groups and is used to power many important cellular processes.
To know more about ATP (adenosine triphosphate) here:
https://brainly.com/question/897553#
#SPJ11
Which type of circulation does the right side of the heart provide?
a. pulmonary
b. cerebral
c. extremity
d. systemic
Answers
The right side of the Heart provides Pulmonary Circulation.
What is a Heart?
The main organ of your circulatory system, a web of blood veins that circulates blood throughout your body, is your heart. Your heart rate and blood pressure are also controlled by other bodily systems.
The heart is a type of pumping machine that is used to pump blood through the vessels of your body. This includes carrying out oxygen and nutrients in the body and carrying out carbon dioxide from the body.
Therefore the heart is a very important part of our body.
To learn more about Heart from the given link
https://brainly.com/question/16566688
#SPJ4
How many ions would you expect MgBr2 to break down into in water?2314
Answers
When MgBr2 is dissolved in water, it would break down into three ions.MgBr2 is an ionic compound consisting of one magnesium cation (Mg2+) and two bromide anions (Br-).
When MgBr2 is dissolved in water, the water molecules surround and separate the ions from each other. This process is known as hydration. The magnesium cation has a charge of +2, while the bromide anions have a charge of -1 each. Therefore, to achieve overall electrical neutrality, two bromide ions are required for every magnesium ion.
In water, the magnesium cation and bromide anions dissociate from the solid compound and become hydrated, surrounded by water molecules. Since there are two bromide anions per magnesium cation in MgBr2, the compound would dissociate into three ions: one magnesium ion (Mg2+) and two bromide ions (2Br-). Therefore, when MgBr2 is dissolved in water, it would break down into three ions.
Find more about MgBr2
brainly.com/question/27249833
#SPJ4
Calculate the mass of sodium acetate trihydrate required to make 100. Ml of a 0. 100 m solution. As part of your preparation for this experiment, record your answer to this question in your notes so that you have it ready to reference during the lab session. If you do not get this question correct, please make sure to revisit the calculation and check your corrected value with your ta prior to the lab session.
Answers
The mass of sodium acetate trihydrate required to make 100 mL of a 0.100 M solution is 0.100 grams. To make a 0.100 M solution, 0.100 moles of sodium acetate trihydrate is required for every liter of solution.
As a result, we can calculate the required mass of sodium acetate trihydrate by multiplying the number of moles by the molar mass of sodium acetate trihydrate, which is 82.03 g/mol. Sodium acetate trihydrate (CH3COONa•3H2O) is a white crystalline solid that is commonly used in various applications, including in food production as a flavor enhancer and in the laboratory as a buffer solution.
To make a 0.100 M solution, 0.100 moles of sodium acetate trihydrate is required for every liter of solution. To find the number of moles, we can multiply the concentration by the volume: 0.100 M x 0.100 L = 0.0100 moles. The molar mass of sodium acetate trihydrate is 82.03 g/mol, so we can multiply the number of moles by the molar mass to find the required mass: 0.0100 moles x 82.03 g/mol = 0.100 grams.
Learn more about sodium acetate trihydrate:
https://brainly.com/question/8445600
#SPJ4
what is the formular of ammonia
Answers
NH3
Why are you using brainly for this simple question? Search history being checked?
Answer:
[tex]NH_{3}[/tex]
Explanation:
Structure and Formula of Ammonia
Its chemical formula is NH3 and the molar mass is 17.03 g/mol. It is a compound of hydrogen and nitrogen.
How do you calculate pH from KOH?
Answers
To calculate the pH from KOH, you need to first determine the concentration of the hydroxide ion (OH-) in the solution.
This can be done by using the equation:
[OH-] = [KOH]
Once you have the concentration of the hydroxide ion, you can use the equation:
pOH = -log[OH-]
to find the pOH of the solution. Finally, you can use the equation:
pH = 14 - pOH
to find the pH of the solution.
Here is a step-by-step explanation:
1. Determine the concentration of the hydroxide ion (OH-) in the solution by using the equation:
[OH-] = [KOH]
2. Use the equation:
pOH = -log[OH-]
to find the pOH of the solution.
3. Use the equation:
pH = 14 - pOH
to find the pH of the solution.
By following these steps, you can calculate the pH from KOH.
To know more about pH here:
https://brainly.com/question/15289741#
#SPJ11
describe how you could use the liquid's physical and chemical properties to determine what liquid it might be
Answers
We would look at the liquid's solubility as well as boiling point to establish the kind of liquid based on its physical and chemical qualities.
What is liquid?One of the four main states of matter is liquid, the others who were solid, gas, and plasma. A liquid is a form of fluid. In contrast to a solid, those molecules found in a liquid possess significantly more flexibility to move.
The forces that hold molecules together within a solid are really only transient in a liquid, permitting it to flow while still being a solid. We would look at the liquid's solubility as well as boiling point to establish the kind of liquid based on its physical and chemical qualities.
Therefore, we would look at the liquid's solubility as well as boiling point to establish the kind of liquid based on its physical and chemical qualities.
To know more about liquid, here:
https://brainly.com/question/20922015
#SPJ9
Your question is incomplete but most probably your full question was,
Describe how you could use the liquid's physical and chemical properties to determine what liquid it might be? solubility, melting point, boiling point
What is the chloride lewis dot structure?
Answers
The Lewis dot structure of chloride ion (Cl-) is represented by a single dot on the outermost shell of Cl.
The chloride particle is an adversely charged particle made out of one chlorine molecule. The Lewis spot design of chloride particle is addressed by the image Cl−, which has a solitary speck on its peripheral shell. The spot addresses a valence electron that isn't associated with holding with different iotas. Chlorine has seven valence electrons, yet it needs another electron to finish its octet, so it acquires one electron from one more particle to shape the chloride particle. In the Lewis speck structure, the chloride particle is encircled by eight dabs, addressing the full octet of electrons. The chloride particle is in many cases found in ionic mixtures like sodium chloride (NaCl).
To learn more about lewis dot structure, refer:
https://brainly.com/question/20300458
#SPJ4
What mass of NaCl is present in a 1.05 m NaCl solution that has 250. g solvent? a) 24.5 g b) 4.49 g c) 6.54 g d) I DON'T KNOW YET
Answers
The mass of NaCl present in the solution is 15.32 g. Answer (d) was not given as a choice, but it is the correct answer based on the calculation.To calculate the mass of NaCl in the solution, we need to first calculate the amount of NaCl present in the solution, which can be found using the formula:
amount of solute (in moles) = concentration x volume (in liters)
We know that the concentration of the solution is 1.05 m, and the volume of the solvent is 250 g, which is equal to 0.25 L (assuming the density of the solvent is 1 g/mL). Therefore, the amount of NaCl in the solution is:
amount of NaCl = 1.05 m x 0.25 L = 0.2625 moles
Next, we need to calculate the mass of NaCl using its molar mass, which is 58.44 g/mol. Therefore, the mass of NaCl in the solution is:
mass of NaCl = 0.2625 moles x 58.44 g/mol = 15.32 g
Therefore, the mass of NaCl present in the solution is 15.32 g. Answer (d) was not given as a choice, but it is the correct answer based on the calculation.
Find out more about amount of NaCl
brainly.com/question/14836855
#SPJ4
What is the definition of reaction time?
Answers
Reaction time is the amount of time it takes for a person or a system to respond to a stimulus or a change in the environment. In other words, it is the time interval between the onset of a stimulus and the initiation of a response.
Reaction time can be measured in different contexts, such as in human performance, neuroscience, sports, and engineering. For example, in human performance, reaction time is often used to assess cognitive and motor skills, such as reaction time in driving, reaction time in decision-making, or reaction time in sports.
In neuroscience, reaction time is used to study neural processing and the speed of information processing in the brain. Reaction time can be influenced by various factors, such as age, gender, attention, arousal, motivation, and the complexity of the task.
Reaction time can be improved with practice and training, and it can also be affected by various diseases and disorders, such as Parkinson's disease, multiple sclerosis, or attention deficit hyperactivity disorder (ADHD).
To know more about Reaction time here
https://brainly.com/question/13693578
#SPJ4
in the common lab measurements experiment, how is the crucible heated before adding the hydrated sulfate?
Answers
In the common lab measurements experiment, the crucible is typically heated using a Bunsen burner or a hot plate before adding the hydrated sulfate.
What is a Bunsen burner?
A Bunsen burner is a common laboratory tool used for heating, sterilization, and combustion. It consists of a metal base with a vertical metal tube that has an adjustable air intake and gas valve. The gas valve controls the amount of gas that is allowed to enter the burner, while the air intake controls the amount of oxygen that is allowed to mix with the gas.
The goal is to heat the crucible to a high temperature to remove any moisture or impurities that may be present on the surface of the crucible. This ensures that the mass of the crucible is accurately measured before and after the addition of the hydrated sulfate.
To heat the crucible using a Bunsen burner, the burner is placed under a tripod or ring stand, and the crucible is placed on a clay triangle or wire gauze over the flame. The burner is adjusted to produce a hot, blue flame, and the crucible is heated for several minutes until it glows red.
To heat the crucible using a hot plate, the hot plate is turned on and set to a high temperature, and the crucible is placed on the plate. The hot plate heats the crucible evenly, and the temperature can be adjusted as needed to achieve the desired level of heat.
After the crucible has been heated, it is allowed to cool to room temperature before the hydrated sulfate is added.
Hence, a Bunsen burner is used to heat the crucible.
To learn more about a Bunsen burner:
https://brainly.com/question/10281181
#SPJ4
Which of these ions is most likely to be leached from the soil?
a. magnesium ions,
b. chlorine ions,
c. calcium ions,
d. iron ions, or
e. potassium ions
Answers
Options e, Potassium ions are most likely to be leached from the soil.
The ion that is most susceptible to being solubilized from soil depends on the chemistry of the soil, the amount of moisture in the soil, and whether or not any plants may absorb particular ions.
Positively charged ions like potassium, magnesium, as well as calcium may often be leached out of the soil when it's moist and migrates into the groundwater along with the water.
However, due to their pH levels, mineral makeup, and other characteristics, some soils are more prone to the leaching of particular ions.
Learn more about soil at
https://brainly.com/question/23813511
#SPJ4
Is sucrose a reducing or non-reducing sugar?
Answers
Sucrose is a non-reducing sugar.
A reducing sugar is a sugar that has a free aldehyde or ketone functional group, which can reduce certain chemicals such as Benedict's solution and Fehling's solution. On the other hand, non-reducing sugars do not have free aldehyde or ketone groups and, therefore, cannot reduce these chemicals.
Sucrose is a disaccharide made up of glucose and fructose, which are both reducing sugars on their own. However, when they are joined together by a glycosidic linkage, the aldehyde group of glucose and the ketone group of fructose are bonded together, forming a non-reducing disaccharide. Therefore, sucrose cannot reduce Benedict's or Fehling's solution and is considered a non-reducing sugar.
To know more about the sucrose, here
brainly.com/question/29186350
#SPJ4
How many grams of iron are produced when 220 g of iron(III) oxide (Fe2O3) are reacted? Show work
Fe2O3+3 H₂--> 2Fe + 3H₂O
1 mol Fe₂03-159.6 g Fe2O3
1 mol Fe=55.8 g Fe
Answers
the amount of the iron that is produced was found to be 195.8 g of iron will be produced.
write the balanced equation of the iron oxide ?
According to the balanced chemical equation:
Fe2O3 + 3H2 → 2Fe + 3H2O
We can see that 1 mole of Fe2O3 reacts to produce 2 moles of Fe. We can use this ratio to convert the given mass of Fe2O3 to the mass of Fe produced.
1 mol Fe2O3 = 159.6 g Fe2O3
220 g Fe2O3 x (1 mol Fe2O3/159.6 g Fe2O3) x (2 mol Fe/1 mol Fe2O3) x (55.8 g Fe/1 mol Fe) = 195.8 g Fe
To solve this problem, we first need to use stoichiometry to determine how much iron (Fe) will be produced from the given amount of iron(III) oxide (Fe2O3).
Therefore, 195.8 g of iron will be produced.
To learn more about iron follow the given link: https://brainly.com/question/14964747
#SPJ1
What is the clf5 lewis structure?
Answers
Explanation:
- Clf5 Lewis Structure.
What is the molecular geometry and molecular polarity for OF2?
Answers
The molecular geometry of Oxygen Difluoride molecule, OF₂ is bent shape and the in case of molecular polarity, it is a polar molecule.
We have a Oxygen Difluoride molecule with molecular formula,OF₂. It consists two fluorine atoms and one oxygen atom.
molecular geometry of OF₂The Lewis structure of the OF₂ molecule contains 16 non-bonding electrons, i.e. 8 lone pairs. Out of the 8 lone pairs, 3 lone pairs are present on the fluorine atom and 2 lone pairs are present on the central oxygen atom. The presence of lone pairs on the oxygen atom causes the electrons of the bonded pairs to repel each other. Due to the repulsive forces, the fluorine which is present as outer atoms pushes down to minimize the repulsion according to the VSEPR theory. So, the shape of this molecule is bent.
Molecular polarityIn the OF₂ molecule, the oxygen atom is the least electronegative, i.e., 3.44 and fluorine is highly electronegative, i.e., 3.98. The difference between the electronegativities of both these atoms is greater than 0.5, making the O-F bonds polar. So both single bonds formed between oxygen and fluorine atoms are polar.
For more information about Molecular geometry, refer:
https://brainly.com/question/19354582
#SPJ4
One mole of the ionic compound, NaCl, will dissolve into _ _ _ _ _ _ _ moles of particles in aqueous solution.
Answers
One mole of the ionic compound NaCl will dissolve into two moles of particles in aqueous solution.
When NaCl dissolves in water, it dissociates into its constituent ions, Na+ and Cl⁻. Each formula unit of NaCl dissociates into one Na⁺ ion and one Cl⁻ ion, so one mole of NaCl will produce one mole of Na⁺ ions and one mole of Cl⁻ ions, for a total of two moles of particles.
It's important to note that not all ionic compounds will dissociate into two ions like NaCl does. Some ionic compounds, like CaCO₃ (calcium carbonate), will only dissociate partially into ions in solution, while others may dissociate into more than two ions. However, for NaCl specifically, one mole will dissociate into two moles of particles in solution.
To know more about the NaCl, here
brainly.com/question/18248731
#SPJ4
particles of electromagnetic radiation are called ______, and each has a discrete amount of energy called a ______. since electromagnetic radiation also has wave properties, each particle is also characterized by a specific ______ and frequency.
Answers
Particles of electromagnetic radiation are called photons, and each has a discrete amount of energy called a quantum or a photon energy. Since electromagnetic radiation also has wave properties, each photon is also characterized by a specific wavelength and frequency.
According to the equation E = hf, where E is the energy of a photon, h is Planck's constant, and f is the photon's frequency, the energy of a photon is directly proportional to its frequency and inversely proportional to its wavelength.
A type of energy that moves through space at the speed of light is electromagnetic radiation. It is made up of oscillating magnetic and electric fields that are parallel to the wave's propagation direction and to each other.
Magnetic field radiation can have
Learn more about electromagnetic radiation here:
https://brainly.com/question/10759891
#SPJ4
When heating this reaction mixture at reflux, the reaction temperature will be maintained at approximately. A. 25C B. 65C C. 100C D. 125C.
Answers
The correct answer is C. the reaction temperature will be maintained at approximately 100°C.
When a reaction mixture is heated at reflux, the temperature of the mixture will be maintained at the boiling point of the solvent.Reflux is a process that involves condensing vapours and transferring the resulting condensate back to the system it originally came from. It is employed in both commercial and academic distillations. Moreover, it is employed in chemistry to provide long-lasting reactions with energy. For example, if the reaction mixture is heated with water, the temperature will be kept at 100°C. Therefore, when heating a reaction mixture at reflux, the reaction temperature will be maintained at approximately 100°C.
learn more about Reflux Refer:brainly.com/question/28261084
#SPJ4
is sugar ionic or covalent
Answers
The sugar is the covalent compound. The sugar is composed of the carbon, the oxygen, and the hydrogen .
The Sugar, are composed of the carbon, the oxygen, and the hydrogen and has the covalent bonds. The covalent bond is formed when the bond is formed by the mutual sharing of the electrons in between the atoms or the molecules.
The compound formed by the covalent bond is called as the covalent compounds. Therefore, the sugar is the covalent compound as all the covalent bonds in the sugar molecules arise by the result of the electron sharing in between the atoms.
To learn more about covalent here
https://brainly.com/question/30420584
#SPJ4
Of all the species that enzymes bind, they are thought to bind most tightly to _____. a) substrates b) products c) transition states d) intermediates
Answers
It is believed that Transition states are the species that enzymes bind to most strongly out of all other species.
What is meant by Enzyme?The ability of an enzyme's active site to connect to its substrate or substrates specifically with the help of certain amino acids can speed up chemical reactions.An enzyme will attach to (bind) one or more reactant molecules in order to catalyze a process. These substances are the substrates for the enzyme.One substrate may split into several products in various reactions. The byproducts then depart from the enzyme's active site.The biochemical reactions in living things are sped up by enzymes, which are biological catalysts. Enzymes have little impact on the process' equilibrium.The substrate is transformed into the product by the enzymes. The enzymes create the enzymes substrate complex when they bind to the active site of the substrate.To learn more about Enzyme, refer to:
https://brainly.com/question/2536131
#SPJ4
the colligative molality of seawater is approximately 1.10 m. calculate the vapor pressure of sea water at 20 °c. the vapor pressure of pure water at 20 °c is 17.54 torr.
Answers
the colligative molality of seawater is approximately 1.10 m, the vapor pressure of sea water at 20 °c is 16.412 torr, the vapor pressure of pure water at 20 °c is 17.54 torr.
What is vapour pressure?Vapour pressure rises with temperature and is a measurement of a substance's propensity to transform into a gaseous or vapour state. The boiling point of a liquid is the temperature at which the pressure imposed by its surroundings equals the vapour pressure present at the liquid's surface. A liquid's thermodynamic propensity to evaporate is indicated by the equilibrium vapour pressure.
Given that-
Vapor pressure of pure water (P₀) = 17.54 torr
Molality (m) = 1.10 m
Let, vapour pressure of sea water = P
As we know,
m = [(P₀ - P) / P₀] × (1000 / M(solute))
1.10 = [(17.54 - P) / 17.54] × (1000 / 58.44)
P = 16.412 torr [considering NaCl in sea water]
Hence, vapour pressure of sea-water is: 16.412 torr
To know more about colligative molality refer to:
https://brainly.com/question/14611438
#SPJ1
The Bohr effect describes the tendency for hemoglobin to more readily unload oxygen under which conditions?increased pH and decreased PCO2decreased pH and decreased temperatureincreased pH and PCO2decreased pH and increased temperature
Answers
The Bohr effect describes the tendency for a hemoglobin to more readily unload oxygen under decreased pH and increased temperature. Option D is correct.
The Bohr effect describes the tendency for hemoglobin to more readily unload oxygen under decreased pH conditions. This means that when the pH of the blood decreases, hemoglobin is more likely to release its bound oxygen molecules to the tissues that need it. This is a crucial mechanism for the efficient delivery of oxygen to active tissues during periods of increased metabolic demand.
In addition to decreased pH, other factors that can enhance the Bohr effect and increase oxygen release include increased levels of carbon dioxide (PCO₂) and increased temperature. However, increased pH would actually decrease the Bohr effect and decrease the release of oxygen from hemoglobin.
Hence, D. decreased pH and increased temperature is the correct option.
To know more about Bohr effect here
https://brainly.com/question/7017026
#SPJ4
--The given question is incomplete, the complete question is
"The Bohr effect describes the tendency for hemoglobin to more readily unload oxygen under which conditions? A) increased pH and decreased PCO₂ B) decreased pH and decreased temperature C) increased pH and PCO₂ D) decreased pH and increased temperature."--
What is br molar mass ?
Answers
The molar mass of bromine (Br) is 79.904 g/mol.
The molar mass of an element is the mass in grams of one mole of that element. It is equal to the atomic weight of the element in atomic mass units (amu) and is expressed in grams per mole (g/mol).
Molar mass can be found on the periodic table of elements, where the atomic weight of each element is listed.
To calculate the molar mass of a compound, you simply add up the molar masses of each element in the compound. For example, the molar mass of HBr (hydrogen bromide) would be the molar mass of hydrogen (1.008 g/mol) plus the molar mass of bromine (79.904 g/mol), which equals 80.912 g/mol.
The molar mass of an element or compound is an important concept in chemistry that allows us to relate the mass of a substance to the number of moles of that substance.
To know more about molar mass here:
https://brainly.com/question/30216315#
#SPJ11