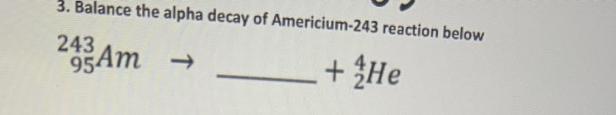Answers
Answer:
²⁴³₉₅Am --> ²³⁹₉₃Np + ⁴₂He
Explanation:
In alpha decay;
The mass number decreases by 4
Atomic Number decreases by 2
Mass Number = 243 - 4 = 239
Atomic Number = 95 - 2 =93
Element = Neptunium (Np)
Equation for the decay is given as;
²⁴³₉₅Am --> ²³⁹₉₃Np + ⁴₂He
Related Questions
It late, I need help quick
Answers
Answer:
what is late ? there is no attachment ?
Answer:
For people asking for the questions
Explanation:
HELP WITH A THESIS STATEMENT
I basically need a thesis statement on how DNA analysis relates to chemistry. The only problem is that I'm having trouble making it 'arguable'. My ideas were:
-DNA analysis relates to chemistry because it applies that knowledge when analyzing the DNA samples left at crime scenes in order to identify the suspect.
-DNA analysis relates to chemistry because it's a subcategory of chemistry, also known as forensic chemistry, where DNA left at crime scenes is analyzed to potentially link a suspect to a crime.
Do either of these sound good and are they arguable? If not can you reword it to be?
Answers
The second thesis statement is perfect. It supports the claim and presents main idea.
Electrical energy exists when charged particles attract or repel each other.
PLEASE HELP
True or false
Answers
I think true..
Explanation:
im not sure.
Give examples which indicate that nylon fibres are very strong.
Answers
Explanation:
is used for making ropes, used for climbing rocks and for making parachutes. Their usage shows that nylon fibres have high tensile strength
According to Banquo, the King "Sent forth great largess to your offices." What
gift does King Duncan bring for Lady Macbeth?
Answers
Answer:
Diamond
Explanation:
The answer is gotten from Act 2, Scene 1 of the play by Shakespeare titled "Macbeth". In this scene, we see that Banquo met Macbeth late in the night where they had a profound conversation about the gift of diamond which king Duncan had given to Banquo to deliver to Macbeth.
Question 1
Which of the following is a false
Answers
Answer:
1.A 2.C 3.B
4. d
Explanation:
which of the following has more particles
6.02x10(small numbers)23 molecules CO2
9 moles PF2
10 mole NaCI
18 g H2O
Answers
Answer:
A mole is Avogadro's number of items: 6.022 × 1023.
Explanation:
A bike tire at a pressure of 740 mmHg and a
temperature of 290 K has a volume of 2.2 liters.
A child gets on the bike and rides it outside.
What is the new pressure if the temperature
outside is 296 K and his weight causes the tire's
volume to drop to 2.0 liters?
Answers
Answer: The new pressure is 831 mm Hg
Explanation:
The combined gas equation is,
[tex]\frac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2}[/tex]
where,
[tex]P_1[/tex] = initial pressure of gas = 740 mm Hg
[tex]P_2[/tex] = final pressure of gas = ?
[tex]V_1[/tex] = initial volume of gas = 2.2 L
[tex]V_2[/tex] = final volume of gas = 2.0 L
[tex]T_1[/tex] = initial temperature of gas = 290 K
[tex]T_2[/tex] = final temperature of gas = 296 K
Now put all the given values in the above equation, we get:
[tex]\frac{740\times 2.2}{290}=\frac{P_2\times 2.0}{296}[/tex]
[tex]P_2=831mmHg[/tex]
The new pressure is 831 mm Hg
True or false The force of gravity acting on an object is measured by weight.
Answers
Answer:
true
Explanation:
we need gravity to stay on ground
Answer:
True.
Explanation:
Weight in a physics or scientific sense is defined mathematically as "mass times gravity." It is literally a measure of the force of gravity pulling mass downward towards Earth (or whatever object you're considering).
What determines the type of bond (single, double, or triple) an element will form?
Answers
The type of bond formed depends on the valence electrons (no: of electrons in the outermost shell)
When two atoms share one electron pair between each other, then they are said to be bonded by single covalent bond
When two atoms share two electron pairs between each other, they are said to be bonded by double covalent bond
When two atoms share three electron pairs between each other, they are said to be bonded by triple covalent bond.
Hope u understand
Please mark as the brainliest
I need help! Please & Thank you!
Answers
Answer:
I think it is B
Explanation:
1. A sample of naproxen has 6.022x10^23 naproxen molecules. How many moles is this?
Answers
Answer:
0.99997661 moles
Explanation:
Answer:
ysys7sbssiej hsbed hdbd hdbd
what would happen if organ systems failed to work together
Answers
Answer:
If the organ systems do not work together, then homeostasis is not maintained. If homeostasis is not maintained, the cells of the body will not work properly. As a result, the body will not work properly.Feb 4, 2017
Explanation:
Answer:
If the organ systems do not work together, then homeostasis is not maintained. If homeostasis is not maintained, the cells of the body will not work properly. As a result, the body will not work properly.
Explanation: Edge 2020 :)
Which of the following statements is true?
Answers
the answer is the third one
Explanation:
it just is I think
Which statement is not
true?
A. The troposphere is responsible for nearly
all of Earth’s weather.
B. The exosphere reaches deep into space
and is the least dense layer.
C. The thermosphere typically breaks up
meteors before they hit Earth.
D. The stratosphere allows commercial
airlines to fly with less turbulence because
of fewer convection currents.
Answers
explanation: to make C correct, the sphere that breaks up meteors is the mesosphere.
The atmosphere of the earth is layered and each layer of the atmosphere has its own properties. The atmosphere of the earth is divided into four layers. The thermosphere typically breaks up meteors before they hit Earth is not correct. The correct option is C.
What is mesosphere?The four important layers of atmosphere are troposphere, stratosphere, thermosphere and mesosphere. The layer of the atmosphere which is found above the stratosphere is defined as the mesosphere. It is the coldest layer of the atmosphere.
A meteor is known as a celestial object which is made up of rocks and minerals which enters the atmosphere of the earth and burns out completely before reaching the surface of the earth. It is known as the shooting star or falling star.
The temperature of mesosphere drops with altitude. By 80 km it reaches 100 degree celsius. The meteors burn up in this layer not in thermosphere.
Thus the correct option is C.
To know more about earth layers, visit;
https://brainly.com/question/13497783
#SPJ3
How many moles of MgO are produced if 0.37 moles of O2 react with excess of Mg?
Answers
Answer:
yez so the answer is mg 1.0
Explanation:
The excess reactant is present in an amount that is greater than required. The amount of MgO that is produced in the reaction is 0.74 moles.
What is an excess reactant?An excess reactant is that reantant thatis present in an amount that is more than required.
Now we are told that the magnessium is the reactant in excess so we have to use the amount of oxygen.
The reaction is: 2Mg + O2 ---->2MgO
1 mole of O2 produces 2 mole of MgO
0.37 moles of O2 produces 0.37 moles * 2 moles/1 mole
= 0.74 moles
Learn more about excess reactant: https://brainly.com/question/365923
(Help would be greatly appreciated) What is the molarity of 3 moles of HCl in 3 L of water?
1. 3M
2. 1M
3. 6M
4. 9M
Answers
Answer:
1 M
Explanation:
Molarity is moles per liter (mol/L).
You have 3 moles of HCl in 3 L of water. Divide the two values.
(3 mol)/(3 L)
= 1 mol/L
= 1 M
The chemical equation below represents a reversible reaction.
H2PO4- + H2O ↔ H3PO4 + OH-
Which pair represents an acid and its conjugate base?
Answers
Answer:b
Explanation:
bc
In the given chemical equation, H₃PO₄ is an acid while it's conjugate base is OH⁻.
What is chemical equation?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equation,here:
https://brainly.com/question/28294176
#SPJ3
If I have 0.275 moles of gas at a temperature of 75 K and a pressure of 1530 mmHg, what is the volume of the gas?
Answers
Answer: The volume of the gas is 0.84 Liters
Explanation:
According to ideal gas equation:
[tex]PV=nRT[/tex]
P = pressure of gas = 1530 mm Hg = 2.01 atm (760mmHg=1atm)
V = Volume of gas = ?
n = number of moles = 0.275
R = gas constant =[tex]0.0821Latm/Kmol[/tex]
T =temperature =[tex]75K[/tex]
[tex]V=\frac{nRT}{P}[/tex]
[tex]V=\frac{0.275atm\times 0.0820 Latm/K mol\times 75K}{2.01atm}=0.84L[/tex]
Thus the volume of the gas is 0.84 Liters
Which of these compounds has the largest percentage by mass of nitrogen? NO N2O NO2 N2O3
Answers
The largest mass percentage of nitrogen in NO. Therefore, option (A) is correct.
What is the mass percentage?The percentage by mass of an element in a chemical compound can be described as the number of parts by mass of the element contained in 100 parts by mass of a chemical compound.
The mass percentage of an element can be determined in two steps:
First, calculate the molar mass of the compound and then the percentage of each element by dividing the total mass of the element present in the compound by the molecular mass of the compound multiplied by 100.
Given, the molecular formula of the compound is NO.
The molecular mass of the NO = 30 g/mol
The mass percentage of the Nitrogen = (14/30) × 100 = 46.67 %
the molecular formula of the compound is NO₂.
The molecular mass of the NO = 46 g/mol
The mass percentage of the Nitrogen = (14/46) × 100 = 30.4 %
the molecular formula of the compound is N₂O.
The molecular mass of the NO = 44 g/mol
The mass percentage of the Nitrogen = (14/44) × 100 = 31.81 %
the molecular formula of the compound is N₂O₃.
The molecular mass of the NO = 76 g/mol
The mass percentage of the Nitrogen = (28/76) × 100 = 36.84 %
Learn more about the mass percentage, here:
brainly.com/question/16750428
#SPJ2
5. How many Earths could fit between the Earth and the sun?
30
400
1200
12000
Please please please please help me
Answers
You need to multiply the size of the Earth and 12000
Once you do that you get the distance between the Earth and the Sun
Which statement describes the differences between chemical reactions and nuclear decay rates? Chemical reaction rates vary, but nuclear decay rates are constant. Both chemical reaction rates and nuclear decay rates vary. Nuclear decay rates vary, but chemical reaction rates are constant.
Answers
Answer:
Answer the last one Nuclear decay rates vary, but chemical reaction rates are constant
Explanation:
Correct me if im wrong
Answer:
Chemical reaction rates vary, but nuclear decay rates are constant.
Explanation:
gradepoint
Does liquid detergent conduct electricity?
Answers
Answer:
Yes, it is a good conductor of electricity.
Explanation:
They allow electric particles to pass through them (the bases).
(Hope this made sense)
The battery gets it's energy from the
Answers
Which of the following processes is NOT a way that carbon could move between the atmosphere and the biosphere?
Question 4 options:
respiration (breathing)
combustion (burning)
deep burial, compaction, and cementation
photosynthesis
Question 5 (2 points)
What is the only natural way that carbon can move OUT of the geosphere?
Question 5 options:
burning of fossil fuels
volcanic eruptions
dissolution
decomposition
Question 6 (1 point)
Which carbon reservoir contains the MOST carbon?
Question 6 options:
fossil fuels
atmosphere
land biomass
rocks
Question 7 (2 points)
Which carbon reservoir changes the quickest and has the biggest direct effect on climate?
Question 7 options:
land biomass
ocean
atmosphere
rocks
Question 8 (2 points)
Which best describes the process of ocean acidification?
Question 8 options:
The ocean absorbs more human-produced CO2, causing the acidity to increase
The ocean releases more human-produced CO2, causing the acidity to decrease
The ocean absorbs more natural CO2, causing the acidity to decrease
The ocean receives more polluted runoff, causing the acidity to increase
Question 9 (2 points)
What is a potential impact of ocean acidification?
Question 9 options:
Animals will have a harder time building their shells
Corals may have a harder time building their skeletons
Ocean ecosystems may suffer, making it harder for humans to get food from the ocean
All of the above
Answers
Answer:
question 1 i believe is c i will put the other answeres in comments
when i finish the test
Explanation:
The carbon cycle plays an important in maintaining carbon balance on the earth.
What is the carbon cycle?The carbon cycle is the cycle by which carbon is recycled between the atmosphere and the geosphere.
In the carbon cycle, the process of recycling carbon do not include deep burial, compaction, and cementation.
Volcanic eruptions are one natural way that carbon can move OUT of the geosphere.
Fossil fuels, atmosphere, land biomass and rocks are all carbon reservoirs, but fossil fuels contains the MOST carbon.
The atmosphere is the carbon reservoir that changes the quickest and has the biggest direct effect on climate.
Ocean acidification occurs because the ocean absorbs more human-produced CO2, causing the acidity to increase.
A potential impact of ocean acidification is that ocean ecosystems may suffer, making it harder for humans to get food from the ocean.
Therefore, the carbon cycle is important in maintaining carbon balance on the earth.
Learn more about carbon cycle at: https://brainly.com/question/25845923
What holds more salt warm or cold water?
Answers
Which of the following is an example of acceleration?
A. A boat sits on a boat trailer.
B. A car moves in a straight line at 60 km/hr.
C. A plane moves in air at a steady speed of 850 km/hr.
D. A bus moves on a straight road and then
makes a right turn.
Answers
Answer:
it's either B. or C.. hope this helps!
Explanation:
Answer:
D. A bus moves on a straight road and then makes a right turn.
Explanation:
Acceleration is a change in direction or speed. All of the other examples involve straight lines or constant speed.
For the reaction ?H2 + ?O2 → ?H2O,
What is the maximum amount of H2O which could be formed from 15.52 mol of H2 and 17.95 mol of O2? Answer in units of g.
Answers
Explanation:
H2O, what is the maximum amount of H2O which could be formed from 10.9 g of H2 and 16.03 g ofO2? Answer in units of g. ... 0.500 mol O2 x 2 mol H2O / 1 mol O2 x 18 g H2O/mol H2O = answer in ..
What is the mass in grams of 0.250 mol CH2Cl2?
Answers
Answer:
21.2 grams CH2Cl2
Explanation:
Number of moles: .25
Molar mass of CH2Cl2: 84.93 g/mol
Mass in grams: 84.93(0.250) = 21.2325
Considering the definition of molar mass, the mass of 0.250 moles of CH₂Cl₂ is 21.225 grams.
Definition of molar massThe molar mass of substance is a property defined as its mass per unit quantity of substance, in other words, molar mass is the amount of mass that a substance contains in one mole.
The molar mass of a compound (also called Mass or Molecular Weight) is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of CH₂Cl₂In this case, you know the molar mass of the elements is:
C= 12 g/moleCl= 35.45 g/moleH= 1 g/moleSo, the molar mass of the compound CH₂Cl₂ is calculated as:
CH₂Cl₂= 12 g/mole + 2× 1 g/mole + 2× 35.45 g/mole
Solving:
CH₂Cl₂= 84.9 g/mole
Mass of 0.250 mol CH₂Cl₂Next, you can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 84.9 grams, 0.250 moles of the compound contains how much mass?
[tex]mass=\frac{0.250 molesx84.9 grams}{1 mole}[/tex]
mass= 21.225 grams
The mass of 0.250 moles of CH₂Cl₂ is 21.225 grams.
Learn more about molar mass:
https://brainly.com/question/5216907
https://brainly.com/question/11209783
https://brainly.com/question/7132033
https://brainly.com/question/17249726
Which equation would be used to calculate the rate constant from initial
concentrations?
Answers
Answer: B
Explanation: I just did it
The equation for calculation of rate constant from initial concentration will be k= rate / [tex][A^{m}] [B^{n}][/tex]
What is rate constant?The proportionality variable in the expression represents the link between both the rate of a reaction as well as the concentrations of the reacting components often called the rate constant, sometimes specific rate constant.
What is equation?
A chemical reaction is represented by a word equation that uses the identities of the substance present.
The equation for calculation of rate constant from initial concentration will be k= rate / [tex][A^{m}] [B^{n}][/tex].
To know more about rate constant and equation.
https://brainly.com/question/20305871
#SPJ2