Answers
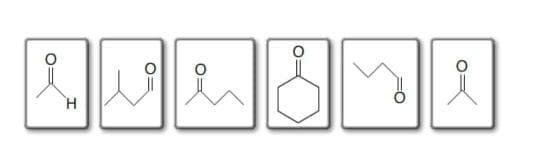
For the following aldehydes and ketones .1 , 4 , 5 are soluble and remaining are not very soluble . ether= CH3CH2OCH2CH3,diethyl ether. Ester= CH3COOCH3, ethyl hexanoate, methyl benzoate, remaining are neither ether nor ester
Aldehydes and ketones are two types of organic compounds that belong to a class of compounds known as carbonyl compounds. The defining feature of these compounds is the presence of a carbonyl group (C=O), which is a carbon atom double-bonded to an oxygen The general structural formula for an aldehyde is R-C(=O)-H, where R can be any organic group. Ketones, on the other hand, have the carbonyl group in the middle of the carbon chain and are characterized by the general structural formula R-C(=O)-R', where R and R' can be any organic group
know more about aldehydes here:
https://brainly.com/question/29756072
#SPJ4
the complete question is:
classify the following aldehydes and ketones as soluble in water or not very soluble in water.
Drag the appropriate items to their respective bins.
Soluble Not very soluble
Related Questions
What mass of water was produced if 350.0 L of carbon dioxide were made at STP? C3H8 (g) + 5O2(g) → 2CO2 (g) + 4H2O (g)
Answers
700 L of water was produced if 350.0 L of carbon dioxide were made at STP.
The quantitative relationship (ratio) between reactants and products in a chemical reaction that produces gases is known as gas stoichiometry. When the created gases are presumed to be ideal and their temperature, pressure, and volume are all known, gas stoichiometry is applicable.
The ideal gas equation is PV=nRT, where n is the number of moles and R is the gas constant, P is the pressure measured in atmospheres (atm), V is the volume measured in liters (L), and
Calculations based on stoichiometry assist scientists and engineers who work in the business world in estimating the number of items they will make using a particular process. They can also assist in determining if a product will be economical to produce.
Reduced growth, reproduction, and survivability for the consumer are typically the results of a significant stoichiometric imbalance between the primary producer and consumer.
To know more about stoichiometry refer to: https://brainly.com/question/9743981
#SPJ1
I need help with my work
Answers
By the mean value theorem, the function y = sin x² on the interval [0, π/4] has this guaranteed number: x ≈ 0.460.
How to find a number that guarantees the mean value theorem
According to the mean value theorem, a function that is differentiable at an interval [a, b] has a number c within the interval such that:
f'(c) = [f(b) - f(a)]/(b - a) (1)
The first derivative of the function is f'(x) = 2 · x · cos x² and by the mean value theorem we know that:
2 · c · cos c² = [sin (π/4)²- sin 0²]/(π/4 - 0)
2 · c · cos c² = [- √2 / 2]/(π /4)
2 · c · cos c² ≈ 0.900
This equation cannot be solved analytically. By the resource of a graphing tool we found the following solution within the interval: c ≈ 0.460.
To learn more on mean value theorem: https://brainly.com/question/1581272
#SPJ1
Suppose certain sample takes 100 min for 750 mL water to percolate into the
soil. Calculate the rate of percolation of water.
Answers
The rate of percolation of water into the soil will be 0.125 mL per second
Percolation rateThe rate of percolation of water into the soil is determined by the volume of water that enters the soil per unit of time under specific conditions.
In this case, 750 mL of water percolated in 100 minutes.
100 minutes = 100 x 60 = 6,000 seconds
Percolation rate = 750/6000 = 0.125 mL per second
More on percolation rate can be found here: https://brainly.com/question/16882186
#SPJ1
grams of hydrogen contains the same number of atoms as 9.79 grams of nitrogen?
Answers
Answer:
1.44 g
Explanation:
1. get number of nitrogen atoms in 9.79 grams of nitrogen
1 mole of Nitrogen is 6.022 x 10^23 atoms N which is = 14.01g N
2.
if 6.022 x 10^23 atoms of Nitrogen is 14
then x atoms of Nitrogen is 9.79
3.
6.022 x 10^23/ x = 14/9.79 ->
x = 8.431 x 10^24 / 9.79 =
8.61799714478 x 10^23
4.
8.61799714478 x 10^23 atoms of N
6.022 x 1023 atoms of H have a mass of 1.008g, so
8.61799714478 x 10^23 atoms of H have a mass of (1.008/6.022 x 1023) x 8.61799714478 x 10^23) = 1.44250051072175g
1.44 g
yeahchemistrycom
Calculate the volume of 32% HCl required to make about 1 N HCl in 100ml volumetric flask
Answers
9.82 mL is the volume of 32% HCl required to make about 1 N HCl in 100ml volumetric flask.
What is Law of Equivalent ?N₁V₁ = N₂V₂
where,
N₁ = Normality of solution with known concentration
V₁ = Volume of solution with known concentration
N₂ = Normality of solution with unknown concentration
V₂ = Volume of solution with unknown concentration
Now find the mass of solution
Mass = Density × Volume
= 1.16 × 0.32 × 1000
= 371.52 g
How to find the Normality ?Normality = [tex]\frac{\text{Weight}}{\text{Equivalent weight}}[/tex]
= [tex]\frac{371.52\ g}{36.5}[/tex]
= 10.18 equivalents
Now put the values in above formula we get
N₁V₁ = N₂V₂
1 × 100 = 10.18 × V₂
100 = 10.18 × V₂
[tex]V_{2} = \frac{100}{10.18}[/tex]
= 9.82 mL
Thus from the above conclusion we can say that 9.82 mL is the volume of 32% HCl required to make about 1 N HCl in 100ml volumetric flask.
Learn more about the Normality here: https://brainly.com/question/9754178
#SPJ1
Balance the equation below and identify the type.
NH4NO₂(s)---> N₂(g) + H₂O(l)
Answers
a 3.211 g sample of gypsum a hydrated salt of magnesuim sulfate, MgSO4.XH2OIS HEATED IN A CRUCIBLE UNTIL A CONSTANT MASS IS REACHED . THE MASS OF THE ANHYDROUS MgSO4 IS 2.539g
a) calculate the percentage by the mass of water in the hydrated MgSO4.xH2O
b) calculate the moles , the amount of H2O released during heating and the amount (in moles) of the anhydrates MgSO4 remaining after heating
c) calculate the value of x in MgSO4.xH2O/ This is the empirical formula of the hydrated magnesium sulfate
Answers
From the calculation, the compound is MgSO4.2H2O.
What is a hydrated salt?A hydrated salt is a salt that contains molecules of the water of crystallization.
a) Percentage of the water of crystallization = 3.211 g - 2.539g/ 3.211 g * 100/1 = 21%
b) Number of moles of water of crystallization = 3.211 g - 2.539g/ 18 g/mol =
0.037 moles
c) Number of moles of anhydrous salt = 2.539g/120 g/mol = 0.021 moles
Number of moles of hydrated salt = 3.211 g/120 + 18x
Hence;
0.021 = 3.211 /120 + 18x
0.021(120 + 18x) = 3.211
2.52 + 0.378x = 3.211
0.378x = 3.211 - 2.52
x = 3.211 - 2.52/ 0.378
x = 2
Hence the compound is MgSO4.2H2O
Learn more about hydrated salt:https://brainly.com/question/5586505
#SPJ1
Boyels law lab report
Answers
Boyle's law is a model used in chemistry and physics to estimate the pressure behavior of gases in a closed system.
What is Boyle's law?Boyle's law is a model used to calculate pressure and volume relationships among gases in a thermodynamically closed system.
This law states that the pressure of a given gas represents an inversely proportional measurement of its volume in steady conditions.
In conclusion, Boyle's law is a model used in chemistry and physics to estimate the pressure behavior of gases in a closed system.
Learn more about Boyle's law here:
https://brainly.com/question/26040104
#SPJ1
Calculate the following using proper units and significant figures: (5.57 m / 0.63 m) - (1.11 m x 0.82 m)
7.93 m
7.931 m
7.9 m²
7.9311 m²
Answers
This will give the value of 7.9311m2
( 5.57m / 0.63m ) - ( 1.11m * 0.82m )
= 8.8413 - ( 1.11m * 0.82m )
= 8.8413 - 0.9102m2
= 7.9311m2
Dividing a value in meter by another value in meter results in the cancellation of both the units and the resulting answer will have no unit.
Multiplying a value in meter by another value in meter it will result in the square meters. It can be written as m2 and can be read a meter square.
Learn more about proper units on
https://brainly.com/question/1622425
#SPJ1
A gas that has a volume of 13 liters, a temperature of 25 0C, and an unknown pressure has its volume increased to 27 liters and its temperature decreased to 15 0C. If I measure the pressure after the change to be 1.3 atm, what was the original pressure of the gas?
Answers
This is an exercise in the general or combined gas law.
To start solving this exercise, we obtain the data:
Data:V₁ = 13 LtT₁ = 25 °C + 273 = 298 kV₂ = 27 LtT₂ = 15 °C + 273 = 288 kP₁ = 1.3 atmP₂ = ¿?We use the following formula:
P₁V₁T₂ = P₂V₂T₁ ⇒ General FormulaWhere
P₁ = Initial pressureV₁ = Initial volumeT₂ = Initial temperatureP₂ = Final pressureV₂ = Final volumeT₁ = Initial temperatureWe clear the general formula for the final pressure.
[tex]\large\displaystyle\text{$\begin{gathered}\sf P_{2}=\frac{P_{1}V_{1}T_{2} }{V_{2}T_{1}} \ \to \ Clear \ formula \end{gathered}$}[/tex]
We substitute our data into the formula to solve:
[tex]\large\displaystyle\text{$\begin{gathered}\sf P_{2}=\frac{(1.3 \ atm)(13\not{l})(288\not{k} )}{(27 \not{l})(298 \not{K})} \end{gathered}$}[/tex]
[tex]\large\displaystyle\text{$\begin{gathered}\sf P_{2}=\frac{4867.2}{8046} \ atm \end{gathered}$}[/tex]
[tex]\boxed{\large\displaystyle\text{$\begin{gathered}\sf P_{2}=0.604 \ atm \end{gathered}$}}[/tex]
If I measure the pressure after the change by 1.3 atm, the original pressure of the gas will be 0.604 atm.
What is indicated by the methyl-prefix?
OA. The molecule is an alkene.
OB. The molecule is a branched hydrocarbon.
O C. The molecule is saturated with hydrogen atoms.
OD. The molecule is a stereoisomer.
Answers
Answer:
B.) The molecule is a branched hydrocarbon.
Explanation:
A hydrocarbon is any molecule made up of carbon and hydrogen exclusively. A methyl- prefix denotes the presence of a methyl group (CH₃), which is situated as a branch off of a hydrocarbon carbon.
Determine the moles of solute in a 350g solution that is 2.5 molal.
Answers
Answer:
0.875 moles solute
Explanation:
You can find the moles of the solute using the molality ratio:
Molality = moles solute / mass (kg) solution
After converting the mass to kilograms, you can plug the values into the ratio and simplify to find the moles.
1,000 g = 1 kg
350 g 1 kg
-------------- x --------------- = 0.35 kg solution
1,000 g
Molality = moles solute / mass solution
2.5 m = moles solute / 0.35 kg
0.875 = moles solute
Carbon monoxide and chlorine gas react to form phosgene:
If a reaction mixture initially contains 215 torr of CO and 245 torr of Cl2, what is the mole fraction of COCl2 when equilibrium is reached?
CO(g) + Cl2(g) ↔COCl2
KP= 310 atm at 700k
Answers
Carbon monoxide and chlorine gas react to form phosgene. If a reaction mixture initially contains 215 torr of CO and 245 torr of Cl₂. 0.76 is the mole fraction of COCl₂ when equilibrium is reached.
How to find Mole fraction ?CO(g) + Cl₂ (g) → COCl₂ (g) Kp = 310
With this reaction we can write the Kp expression
[tex]K_{p} = \frac{p_{COCl_2}}{p_{CO} \times p_{Cl_2}}[/tex]
We know that 1 atm = 760 torr
Now
[tex]p_{CO} = \frac{215 \times 1}{760}[/tex]
= 0.28 atm
[tex]p_{Cl_2} = \frac{245 \times 1}{760}[/tex]
= 0.32 atm
Now, we can write an ICE chart with pressures, so we can know the pressure of COCl₂.
CO(g) + Cl₂ (g) → COCl₂ (g) Kp = 310
I: 0.28 0.32 0
C: -x -x +x
E: 0.28 - x 0.32 - x x
Now put the value in Kp expression
[tex]K_{p} = \frac{p_{COCl_2}}{p_{CO} \times p_{Cl_2}}[/tex]
[tex]310 = \frac{x}{(0.28 - x) (0.32 - x)}[/tex]
[tex]310 = \frac{x}{0.0896 - 0.28x - 0.32x + x^2}[/tex]
310 (0.0896 - 0.28x - 0.32x + x²) = x
310 (0.0896 - 0.6 x + x²) = x
27.776 - 186 x + 310 x² = x
310 x² -187 x + 27.776 = 0
By using the quadratic equation find the value of x:
[tex]x = \frac{b \pm \sqrt{b^2 - 4ac}}{2a}[/tex]
[tex]= \frac{187 \pm \sqrt{(187)^2 - 4 \times 310 \times 27.776}}{2 \times 310}[/tex]
[tex]= \frac{187 \pm \sqrt{34969 - 34,442.24}}{620}[/tex]
[tex]= \frac{187 \pm \sqrt{526.76}}{620}[/tex]
[tex]= \frac{187 \pm 22.95}{620}[/tex]
[tex]= \frac{187 + 22.95}{620}\ \text{or}\ \frac{187 - 22.95}{620}[/tex]
[tex]= \frac{209.95}{620}\ \text{or}\ \frac{164.05}{620}[/tex]
[tex]= 0.33\ \text{or}\ 0.26[/tex]
Using the lowest value of x, we have that the partial pressure of COCl₂ would be 0.26 atm.
PpCO = 0.28 - x = 0.28 - 0.26 = 0.02
PpCl₂ = 0.32 - x = 0.32 - 0.26 = 0.06
[tex]X_{COCl_2} = \frac{P_{COCl_2}}{Pt}[/tex]
And Pt is the total pressure of all species in reaction so:
Pt = 0.02 + 0.06 + 0.26 =
= 0.34 atm
The mole fraction:
[tex]X_{COCl_2} = \frac{0.26}{0.34}[/tex]
= 0.76
Thus from the above conclusion we can say that Carbon monoxide and chlorine gas react to form phosgene. If a reaction mixture initially contains 215 torr of CO and 245 torr of Cl₂. 0.76 is the mole fraction of COCl₂ when equilibrium is reached.
Learn more about the Mole Fraction here: https://brainly.com/question/14498215
#SPJ1
What are the molecular polarity and the intermolecular forces present in the sun
Answers
The molecular polarity of the sun is the dipoole separation of electric charge leading to a molecule in the sun.
What is molecular polarity?Molecular polarity simply refers to those regions of molecules containing or possessing positive and negative charge
So therefore, the molecular polarity of the sun is the separation of electric charge leading to a molecule in the sun.
Learn more about molecular polarity:
https://brainly.com/question/19302084
#SPJ1
Why does Paul Ehrlich concede that the theory of evolution “cannot be refuted by any possible observations” and thus is “outside of empirical science”?
Answers
Evolution can not be tested by experiment given that it depends largely on observations that fit into a wider scope and point to a common conclusion.
Can evolution be proven empirically?Science is know to be empirical, In science, the evidence that backs up a claim is what establishes it as true or false. Thus all theories must be tested by experiment.
However, the theory of evolution can not be tested by experiment given that it depends largely on observations that fit into a wider scope and point to a common conclusion. In other words, evolution lies “outside of empirical science”.
The fact that it can not be directly proven empirically does not necessarily make it out to be false. That was the conclusion of Paul Ehrlich in his 1967 article.
Learn more about evolution:https://brainly.com/question/13492988
#SPJ1
How many MOLES of sulfur hexafluoride are present in 2.33x1022 molecules of this compound
Answers
Answer:
0.0387 moles SF₆
Explanation:
To find the moles of sulfur hexafluoride (SF₆), you need to multiply the number of molecules by Avogadro's number. Avogadro's number is a ratio between molecules and moles. It is important to arrange the conversion in a way that allows for the cancellation of units (the desired unit should be in the numerator). The final answer should have 3 sig figs to match the sig figs of the given value (2.33 x 10²²).
Avogadro's Number:
6.022 x 10²³ molecules = 1 mole
2.33 x 10²² molecules SF₆ 1 mole
--------------------------------------- x ------------------------------------ = 0.0387 moles SF₆
6.022 x 10²³ molecules
An object weighs 32 newtons. What is its mass if a gravitometer indicates that g = 8.25 m/sec2?
Answers
Answer:
3.88kg
Explanation:
weight=mass_of_body(m) x acceleration_due_to_gravity(g)
The weight is given and acceleration is also give
For mass divide the weight by acceleration.
A piece of string is found to be 35.9 meters long. How long is the string in inches?
Answers
Answer: 1413.39 inches
Explanation:
Formula for converting meters to inches
Meters x 39.37 (roughly) = Value in inches
Applying formula
35.9 meters x 39.37 = 1413.39 inches (roughly)
To answer this question, you may need access to the periodic table of elements.
Which of these would likely be the best conductor of electricity?
a.) H₂
b.) NO₂
c.) CaCl₂
d.) H₂O
Answers
Answer:
A
Explanation:
Beacuse the hydrogen is best
The best conductor of electricity here is calcium chloride because, metals are good conductors by the presence of free electrons. The CaCl₂ solution ionizes to Ca²⁺ ions and Cl- ions make it a good electrolyte.
What is conductivity?Conductivity is a physical property which describes the ability of a substance to pass electricity through it. The substance which shows good conductivity at normal temperatures are called conductors and those only conducts at higher temperatures are semiconductors.
Substance which does not conduct in any condition are called insulators. Metals are electron rich and the metal crystal lattice is composed of a sea of mobile electrons and a pool of positive ions. The delocalization of electrons make it easy to pass current through it.
When a metallic solution is ionized, it gives free ions and electrons and make the solution conducting. CaCl₂ is a good conductor since it contains the metal ions Ca²⁺ . Hence, option c is correct.
To find more on conductivity, refer here:
https://brainly.com/question/15085692
#SPJ2
The heat from the bonfire is transferred to the student's hands through the process of
conduction
convection
radiation
reflection
Answers
Answer:
Radiation
Explanation:
Calculate the amount of heat deposited on the skin of a person burned by 1.00g of steam at 100.0C. Skin temperature is 37C. ΔHvap= 40.7kJ/mol.
Answers
The amount of heat deposited on the skin is 2.26 kJ.
What is the amount of heat given off by 1.0 g of steam?The amount of heat given off by steam is determined using the formula below:
Quantity of heat = mass * latent heat of vaporization.
Moles of steam = 1.0/18
Heat = 1.0/18 * 40.7
Heat deposited = 2.26 kJ
In conclusion, the quantity of heat is determined from the latent heat of vaporization and the moles of steam.
Learn more about heat of vaporization at: https://brainly.com/question/26306578
#SPJ1
How many grams of calcium are in 2.25 molar of calcium
Answers
Answer:
40 g
Explanation:b
7. Which of the following compound is the most soluble in CC14? C. NH3 A. HF B. NaCl D. C10H22
Answers
C10H22 is a compound which is the most soluble in CC14 because both are non-polar in nature.
Why polar solute soluble in polar solvent?We know that like dissolve like which means that polar solutes only dissolve in polar solvent while on the other hand, non-polar solutes only dissolve in non-polar solvent.
So we can conclude that C10H22 is a compound which is the most soluble in CC14 because both are non-polar in nature.
Learn more about soluble here: https://brainly.com/question/23946616
#SPJ1
What classifies a substance as an element?
What classifies a substance as a compound?
Actually, answer what the question is asking and give a short answer. No copying and pasting.
Answers
Elements cannot be broken down into simpler substances by any or series of chemical reactions.
A compound can be broken done by chemical reactions
Element and compoundAn element can be defined as species of atoms having a certain number of protons in their nuclei and pure substance of that species.
Elements cannot be broken down into simpler substances by any or series of chemical reactions.
A compound can be defined as a chemical substance that is composed of numerous identical molecules containing atoms from more than one element and held together by chemical bonds.
A compound can be broken done by chemical reactions
Learn more about chemical properties here:
https://brainly.in/question/78631
#SPJ1
what is the mass of 9.3 x 10^ 24 molecules of glucose, C6H12O6 (C6,H12,O6; 180.18 g/mol
Answers
Molecular mass of 9.3 x 10^ 24 molecules of glucose = 1675.6 x 10^ 24
It is given that the mass of glucose is 180.18 g/mol
so if we have to calculate the mass of 9.3x 10^ 24 molecules of glucose
we will apply a simple unitary method i.e,
9.3 x 10^ 24 * 180.18 g/mol = 1675.67 x 10^ 24
To calculate the molecular mass of a molecule, multiply the subscript (number of atoms) by the atomic mass of each element in the molecule and add those masses together.
Remember that - To determine the compound's molecular mass in grams per mole, use the molecular formula.
Divide the supplied mass by the molar mass of the chemical to convert it to moles.
By dividing the number of moles by Avogadro's number, you may convert from moles to molecules.
To learn more about molecular mass please visit -
https://brainly.com/question/18446366
#SPJ1
An acid-base indicatior is a compound who's color is pH dependent. That is, it changes color depending upon whether it is in acid or base. This is because indicators are weak acids and undergo the following equilibrium. H-Ind ↔ H+1 + Ind-1 H-Ind = the indicator molecule that is protonated and predominates under acidic conditions. Ind-1 = the deprotonated indicator molecule (the conjugate base of the acid) and predominates under basic conditions. We are using the Acid-Base indicator, phenopthalein for this experiment. What is the color of this indicator in Acid? . What is the color of this indicator in Base
Answers
Phenolphthalein is pink in acid and colorles in base.
Substances called indicators are those whose solutions change colour as the pH changes. We refer to these as acid-base indicators. The conjugate base or acid versions of these typically weak acids or bases have various hues because of variations in their absorption spectra. When it comes into contact with an object that has a pH of 8.2, it turns pink. At a pH higher than that, it turns purple.
Additionally, ionisation, which modifies the charge and structure of the phenolphthalein molecule, is the cause of this shift in colour. If it comes into contact with an acid like vinegar or a neutral substance like water, it retains its colorlessness.
Therefore, Phenolphthalein is pink in acid and colorles in base.
Learn more about acid - base here;
https://brainly.com/question/12883745
#SPJ1
C3H8O dissolved in water
Answers
If C3H8O is dissolved in water, it would be expected to be a strong electrolyte.
What is a strong electrolyte?
A strong electrolyte is a solute or solution (already an electrolyte) that can completely dissociates in solution.
C3H8O is one of those compounds expected to be a strong electrolyte.
Thus, if C3H8O is dissolved in water, it would be expected to be a strong electrolyte.
Learn more about strong electrolytes here: https://brainly.com/question/2285692
#SPJ1
Which of the following aqueous solutions are good buffer systems?
(Select all that apply.)
a. 0.28 M hydrobromic acid + 0.24 M sodium bromide
b. 0.29 M ammonia + 0.38 M ammonium bromide
c. 0.22 M hypochlorous acid + 0.18 M hydroiodic acid
d. 0.37 M sodium chloride + 0.25 M barium chloride
e. 0.13 M sodium hydroxide + 0.28 M sodium bromide
Answers
0.29 M ammonia + 0.38 M ammonium bromide and 0.22 M hypochlorous acid + 0.18 M hydroiodic acids of aqueous solutions are good buffer systems.
Buffer Systems:A solution that resists pH change when acids or bases are added to it is referred to as a buffer system. Either a weak acid and its salt, or a weak base and its salt, make up buffer systems. The ratio of HX/X- does not considerably alter when an acid or a base is introduced to a buffer.
Solutions known as buffers withstand pH changes when an acid or base is added. A weak base (A) and its conjugate weak acid (HA) are both present in buffers. When a reactive system is in equilibrium, adding a strong electrolyte with one common ion will cause the equilibrium to shift, lowering the concentration of the common ion. Buffers differ from one another in terms of pH range and buffer capacity.
Learn more about buffers here:
https://brainly.com/question/1385846
#SPJ1
Consider the reaction of NaHCO3
with an acid, HA. How many grams of NaHCO3
are required to produce 1.76 g of CO2?
Answers
The mass of NaHCO₃ required to produce 1.76 g of CO₂ is 3.36 g
Balanced equationNaHCO₃ + HA --> NaA + CO₂ + H₂O
Molar mass of NaHCO₃ = 84 g/mol
Mass of NaHCO₃ from the balanced equation = 1 × 84 = 84 g
Molar mass of CO₂ = 44 g/mol
Mass of CO₂ from the balanced equation = 1 × 44 = 44 g
SUMMARY
From the balanced equation above,
44 g of CO₂ were produced from 84 g of NaHCO₃
How to determine the mass of NaHCO₃ neededFrom the balanced equation above,
44 g of CO₂ were produced from 84 g of NaHCO₃
Therefore,
1.76 g of CO₂ will be produced from = (1.76 × 84) / 44 = 3.36 g of NaHCO₃
Thus, 3.36 g of NaHCO₃ is needed for the reaction
Learn more about stoichiometry:
https://brainly.com/question/14735801
#SPJ1
Write an equation for the neutralization of lemon juice with baking soda. Your equation should show a proton transfer to form carbonic acid and a salt.
Answers
The equation of the neutralization reaction is:
HOC(CO₂H)(CH₂CO₂H)₂ + Na₂CO₃ ----> HOC(CO₂Na)(CH₂CO₂Na)₂ + H₂O
What is the acid in lemon juice?The main acid in lemon juice is citric acid.
Citric acid is a tricarboxylic acid and will react with baking soda to form a salt and water.
The equation of the neutralization reaction is shown below:
HOC(CO₂H)(CH₂CO₂H)₂ + Na₂CO₃ ----> HOC(CO₂Na)(CH₂CO₂Na)₂ + H₂O
In conclusion, the neutralization of citric acid in lemon juice produces a salt and water.
Learn more about neutralization reaction at: https://brainly.com/question/15042730
#SPJ1