Answers
Answer:
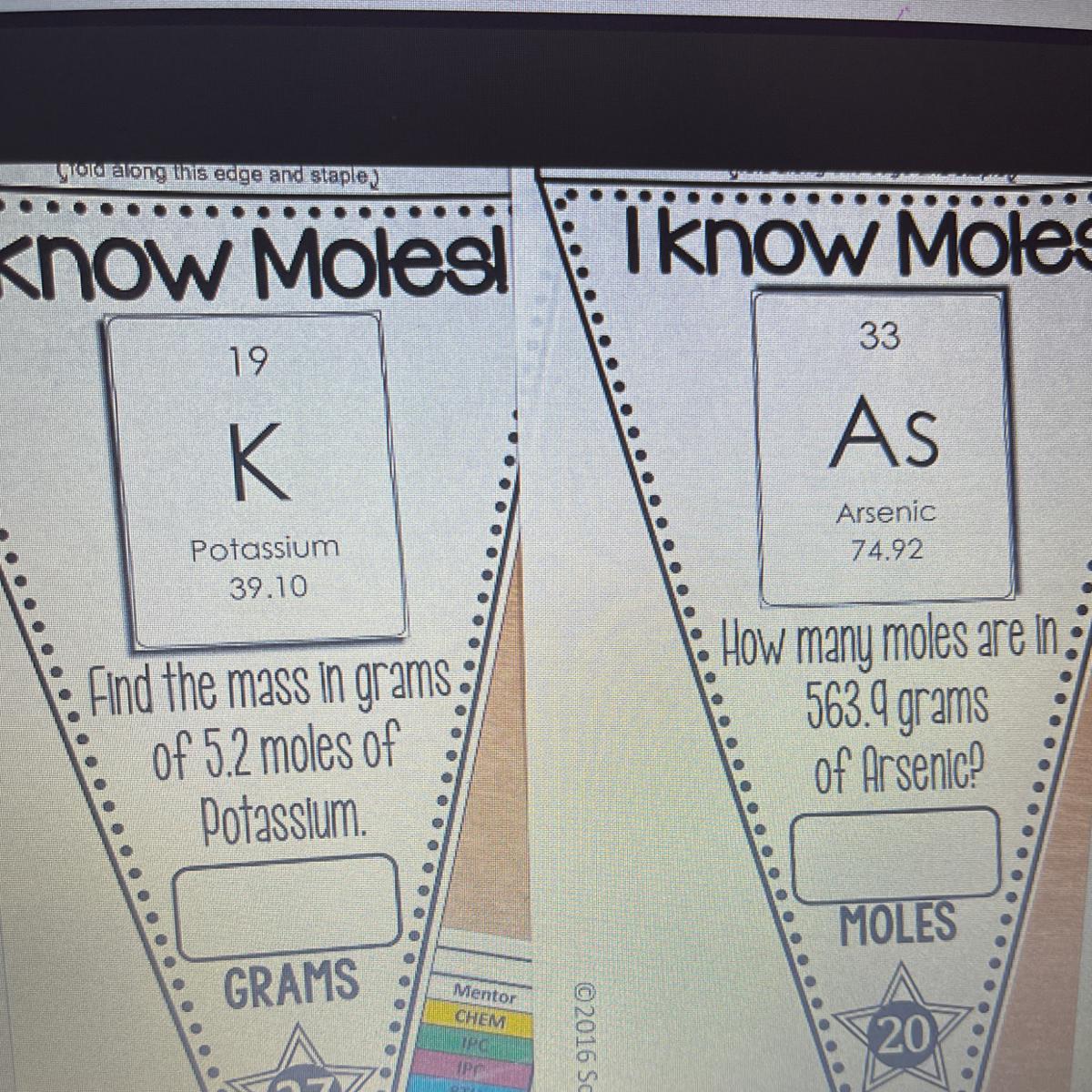
1. Mass of potassium (K) = 203.32 g
2. Number of mole of As = 7.53 moles
Explanation:
1. Determination of the mass of potassium (K)
Molar mass of K = 39.1 g/mol
Number of mole of K = 5.2 moles
Mass of K =.?
Mole = mass / Molar mass
5.2 = mass of K / 39.1
Cross multiply
Mass of K = 5.2 × 39.1
Mass of potassium (K) = 203.32 g
2. Determination of the number of mole of Arsenic (As)
Molar mass of As = 74.92 g/mol
Mass of As = 563.9 g
Number of mole of As =.?
Mole = mass /Molar mass
Number of mole of As = 563.9 / 74.92
Number of mole of As = 7.53 moles
Related Questions
THIS ANSWER CORRECT?
Answers
Answer:
yes
Explanation:
Answer:
im pretty sure! good job :))
Explanation:
im sorry if it isnt in advance, hope this helped a little!
When a chemical change occurs _____.
Need ASAP please someone
Select one:
a. atoms are rearranged
b. the law of conservation of mass is always obeyed
c. the chemical properties of new substances are different from the ones you started with
d. all of these
Answers
Answer:
d
Explanation:
i think its d but im not sure.
Which of these is a likely impact of the stronger than normal trade winds on the eastern Pacific ocean?
Warm surface water builds up, causing lower than average temperature.
Warm surface water builds up, causing higher than average temperature.
Warm surface water is reduced, causing colder conditions than normal.
Warm surface water is reduced, causing hotter conditions than normal.
Answers
Answer:
the answer is c I tink good luck
Answer:
C. Warm surface water is reduced, causing colder conditions than normal.
Explanation:
During El Niño, trade winds are weak. During La Niña, it's the opposite. The surface winds across the entire tropical Pacific are stronger than usual, and most of the tropical Pacific Ocean is cooler than average. Rainfall increases over Indonesia (where waters remain warm) and decreases over the central tropical Pacific
How many moles are in 1.5 x 10^23 atoms of fluorine?
Answers
Answer:
0.052636002587839 moles
Explanation:
im not sure so sorry if u get it wrong...
Baking soda is a critical component of chemical
spill kits. Why would a chemist need baking soda to
help clean up spills?
HELPPPPP NOWWW
Answers
Use the drop-down menus to identify the parts of DNA.
Label A:
Label B:
please help.
Answers
CHEMISTRY HELP! Lewis structure rules
Please help, is this correct?
Answers
Answer:
it is the one below that. NO, because it debt net the octet rule
How many moles are in 64 grams of Ca(NO3
)2
Answers
Answer:
0.39 moles
Explanation:
Given mass of calcium nitrate =64gram
Molar mass of calcium nitrate = 164 gram
No of moles = given mass/ molar mass
No of moles = 64/164
= 0.39 moles
What happens when a gas gets hot
Answers
Heating a gas increases the kinetic energy of the particles, causing the gas to expand. In order to keep the pressure constant, the volume of the container must be increased when a gas is heated.
If the average atomic mass of hydrogen in nature is 1.0079, what does that tell you about the percent composition of H-1 and H-2 in nature
Answers
Answer:
That the isotope H-1 is the most abundant in nature.
Explanation:
Hello!
In this case, since the average atomic mass of an element is computed considering the mass of each isotope and the percent abundance each, for hydrogen we would set up something like this:
[tex]m_H=m_{H_1}*\%abund_{H_1}+m_{H_2}*\%abund_{H_2}[/tex]
Moreover, since the isotope notation H-1 and H-2 means that the atomic mass of H-1 is 1 amu, that of H-2 is 2 amu and the average one is 1.0079 amu, we can infer that the most of the hydrogen in nature is H-1 as the most of it composes the average hydrogen atom.
Best regards!
Which of the following is the definition of erosion?
A the process that involves The movement Of soil and rock
The process Of disitgration Of rock and soil
Neither
Both
Choose one and explain
Answers
Answer:
Neither
Explanation:
None of the answer choices is the correct definition of erosion. Erosion is actually the removal of weathered materials and particle from the surface of rocks and soils.
The process by which rocks and soils are moved is called transportation. The process of disintegration of rocks and soil is called weathering.Erosion is a serious problem in Agriculture as it removes rich and fertile soil cover. This therefore renders the soil barren and stripped of available nutrients.
Answer:
The process of breaking down rock and soil.
Explanation:
A student pushes a textbook across a desk with a force of 6.0 N a distance of 0.3 m. How much work is done on the textbook? Also need the formula and how to do it. Please help
Answers
Explanation:
Work done = Force * Diatance
By applying this equation,
We have Work Done = (6.0N)(0.3m) = 1.8J.
Answer:
1.8 J is the right answer.Explanation:
Formula used : Work = Force × Displacement
Work done = 6.0 Newton×0.3 meters = 1.8 joules
What is true if a liquid and a gas are in equilibrium?
O A Liquid molecules forming a gas and gas molecules forming a
liquid are equal in number.
O B. Liquid molecules are continually forming to replace escaping gas
molecules.
C. The liquid molecules stay liquid, and the gas molecules stay gas.
O D. The number of liquid molecules and the number of gas molecules
vary
Answers
Answer:
A - Liquid molecules forming a gas and gas molecules forming a
liquid are equal in number.
Explanation:
A P E X
How does an objects speed affect its kinetic energy?
Answers
Fill in the blank. Particles in a metal are held together by __________ attractions.
Answers
Particles in a metal are held together by Covalent attractions. These are the strongest attraction or types of bonding
Covalent bonding:The bonds that hold atoms together to form molecules are called covalent bonds. They are pretty tough and not easily made or broken apart. It takes energy to make the bonds and energy is released when the bonds are broken.
For example, there is a covalent bonding between a Chlorine molecule. the two chlorine atoms are held strongly via covalent bonds.
Find more information about Covalent bonding here:
brainly.com/question/11674395
Write a balanced half-reaction for the reduction of gaseous nitrogen N2 to aqueous hydrazine N2H4 in acidic aqueous solution. Be sure to add physical state symbols where appropriate.
Answers
Answer:
Explanation:
N₂ (g ) + 4H⁺( aq ) + 4e⁻ = N₂H₄ ( aq )
H⁺ ion comes from acidic medium . 4 positive charge on proton is balanced by 4 electron to make left hand side neutral because right hand side is neutral .
How does the environment play an important role in creating petrified fossils?
Cold environment prevents fossils from being created.
The right conditions need to be present in order for fossilization to occur.
All fossils are created in the same type of environment.
Hot environment prevents fossils from being created.
Answers
How does the environment play an important role in creating petrified fossils?
Answer:The right conditions need to be present in order for fossilization to occur.
Drier environments, such as land, are more susceptible to the effects of erosion and so it is more difficult to preserve the organism before it decays.
#CARRYONLEARNING #STUDYWELLwhich group name do the non-metals in group 18 have?
Answers
Answer:
The elements of group 18 of the periodic table are known as noble gases or inert gases.
Explanation:
The noble gases are placed in group 18 of the periodic table. These gases have a very low chemical reactivity, that is, little combination with other elements of the periodic table. For that reason they are called inert gases. This behavior is due to its electronic configuration, because its outermost layer or valence layer is always complete, being the stable element by itself, without the need to borrow or share electrons.
As mentioned, noble gases are not very reactive, that is, they do not usually form bonds between atoms. This means that they don't react much with other substances, they don't even react between atoms of the same gas.
In summary, the elements of group 18 are non-reactive elements.
Finally, the elements of group 18 of the periodic table are known as noble gases or inert gases.
I need help asap please help me
Answers
What is the concentration of chloride in a solution made with 0.808 grams of CaCl2 and 250.0 ml of water.?
Answers
Answer:
0.0584 M
Explanation:
From the question given above, the following data were obtained:
Mass of CaCl₂ = 0.808 g
Volume of water = 250 mL
Concentration of chloride =?
Next, we shall determine the number of mole in 0.808 g of CaCl₂. This can be obtained as follow:
Mass of CaCl₂ = 0.808 g
Molar mass of CaCl₂ = 40 + (35.5 × 2)
= 40 + 71
= 111 g/mol
Mole of CaCl₂ =?
Mole = mass / Molar mass
Mole of CaCl₂ = 0.808 / 111
Mole of CaCl₂ = 0.0073 mole
Next, we shall convert 250 mL to L. This can be obtained as follow:
1000 mL = 1 L
Therefore,
250 mL = 250 mL × 1 L / 1000 mL
250 mL = 0.25 L
Next, we shall determine the molarity of CaCl₂. This can be obtained as follow:
Mole of CaCl₂ = 0.0073 mole
Volume of water = 0.25 L
Molarity of CaCl₂ =?
Molarity = mole /Volume
Molarity of CaCl₂ = 0.0073 / 0.25
Molarity of CaCl₂ = 0.0292 M
Finally, we shall determine the concentration of the chloride as illustrated below:
CaCl₂ <=> Ca²⁺ + 2Cl¯
From the equation above,
1 mole of CaCl₂ produced 2 mole of Cl¯.
Therefore, 0.0292 M CaCl₂ will produce = 0.0292 × 2 = 0.0584 M Cl¯.
Thus, the concentration of the chloride ion (Cl¯) in the solution is 0.0584 M
Please help me 17 points!
Answers
Answer:
A dam: 3
solar cells: 2
food: 1
a rocket: 1
Answer:
1: yes
Explanation:
How many moles of H2O are produced from 3 moles of oxygen?
Answers
As you know, the stoichiometric coefficients attributed to each compound in the balanced chemical equation can be thought of as moles of reactants needed or moles of products formed in the reaction. Notice that the reaction requires 2 moles of hydrogen gas and 1 mole of oxygen gas to produce 2 moles of water.
I'm not exactly sure if this is right, but I still hope this helps none the less ^^
6 moles of H2O are produced from 3 moles of oxygen.
What is the amount of moles of H2O produced?
We know that one mole of water is formed from a half mole quantity of oxygen gas so in this way 3 moles of water is produced from using 1.5 moles of oxygen atom so we can conclude that 6 moles of H2O are produced from 3 moles of oxygen.
Learn more about mole here:https://brainly.com/question/1427235
An element with a relatively large amount of electrons in the valence ring is considered to be a good conductor. True False
Answers
Answer:
False
Explanation:
In every substance, there is a valence band and there is a conduction band. The gap between the valence band and the conduction band determines Whether a substance is a conductor or an insulator.
Conductors have a narrow gap between the valence band and the conduction band while insulators have a large gap between conduction band and insulation band. However, elements with a large number of electrons in the valence band are non conductors.
if an exothermic reaction takes place in an insulated container at the end of the reaction the temperature of the content of the container will
Answers
Answer:
Remain the same.
Explanation:
The reaction of the temperature of the content of the container will remain the same because no heat energy is transfer from the container to the external environment due to insulating material of the container. Insulators are poor conductor of heat and electricity so the container is unable to absorb the heat energy produced during a chemical reaction and all energy of the product remain the same.
how is the phenomenon of refraction useful to humanity
Answers
Answer:
Yes, it does have an important role in humanity!
Explanation:
In human life, refraction of light plays an important role. It has many applications in optics and imaging technology. Some of the applications are: Refraction concave and convex glasses are used to correct the refractive errors of human eyes.
its use full to humanity if you stay back jk
Balance the equation.
P + 02 --> P4O10
Answers
Answer:
4P + 5O₂ —> P₄O₁₀
Explanation:
From the question given above, we obtained:
P + O₂ —> P₄O₁₀
The above equation can be balance as illustrated below:
P + O₂ —> P₄O₁₀
There are 4 atoms of P on the right side and 1 atom on the left side. It can be balance by 4 in front of P as shown below:
4P + O₂ —> P₄O₁₀
There are 10 atoms of O on the right side and 2 atoms on the left side. It can be balance by putting 5 in front of O₂ as shown below:
4P + 5O₂ —> P₄O₁₀
Now the equation is balanced.
0.2 mol of hydrocarbons undergo complete combustion to give 35.2 of carbon dioxide and 14.4g of water as the only product. What is the molecular formula of hydrocarbon
Answers
Answer:
[tex]\rm C_4 H_8[/tex].
Explanation:
Look up the relative atomic mass of [tex]\rm C[/tex], [tex]\rm H[/tex], and [tex]\rm O[/tex] on a modern periodic table:
[tex]\rm C[/tex]: [tex]12.011[/tex].[tex]\rm H[/tex]: [tex]1.008[/tex].[tex]\rm O[/tex]: [tex]15.999[/tex].Calculate the molecular mass of [tex]\rm CO_2[/tex] and [tex]\rm H_2O[/tex]:
[tex]M(\mathrm{CO_2}) = 12.011 + 2 \times 15.999 = 44.009\; \rm g \cdot mol^{-1}[/tex];
[tex]M(\mathrm{H_2O}) = 2 \times 1.008 + 15.999 = 18.015\; \rm g \cdot mol^{-1}[/tex].
Given the mass [tex]m[/tex] of [tex]\rm CO_2[/tex] and [tex]\rm H_2O[/tex] produced, calculate the number of moles of molecules that were produced:
[tex]\displaystyle n(\mathrm{CO_2}) = \frac{m(\mathrm{CO_2})}{M(\mathrm{CO_2})} \approx 0.80\; \rm mol[/tex];
[tex]\displaystyle n(\mathrm{H_2O}) = \frac{m(\mathrm{H_2O})}{M(\mathrm{H_2O})} \approx 0.80\; \rm mol[/tex].
Calculate the number of moles of [tex]\rm C[/tex] atoms and [tex]\rm H[/tex] atoms in these [tex]\rm CO_2[/tex] and [tex]\rm H_2O[/tex] molecules.
Each [tex]\rm CO_2[/tex] molecule contains one [tex]\rm C[/tex] atom. Therefore, that [tex]0.80\; \rm mol[/tex] of [tex]\rm CO_2\![/tex] contains [tex]0.80\; \rm mol\![/tex] of [tex]\rm C\![/tex] atoms.Each [tex]\rm H_2O[/tex] molecule contains two [tex]\rm H[/tex] atoms. Therefore, that [tex]0.80\; \rm mol[/tex] of [tex]\rm H_2O\![/tex] contains [tex]2 \times 0.80\; \rm mol = 1.60\; \rm mol[/tex] of [tex]\rm H\![/tex] atoms.The combustion reaction here include two reactants: the hydrocarbon and [tex]\rm O_2[/tex].
As the name suggests, hydrocarbons contain only [tex]\rm C[/tex] atoms and [tex]\rm H[/tex] atoms. On the other hand, [tex]\rm O_2[/tex] contains only [tex]\rm O[/tex] atoms.
Therefore, all the [tex]\rm C[/tex] and [tex]\rm H[/tex] atoms in those [tex]\rm CO_2[/tex] and [tex]\rm H_2O[/tex] molecules are from the unknown hydrocarbon. (With a similar logic, all the [tex]\rm O[/tex] atoms in those combustion products are from [tex]\rm O_2[/tex].)
In other words, that [tex]0.20\; \rm mol[/tex] of this unknown hydrocarbon molecules contains:
[tex]0.80\; \rm mol[/tex] of [tex]\rm C[/tex] atoms, and [tex]1.60\; \rm mol[/tex] of [tex]\rm H[/tex] atoms.Hence, each of these hydrocarbon molecules would contain [tex](0.80\; \rm mol) / (0.20\; \rm mol) = 4[/tex] carbon atoms and [tex](1.60\; \rm mol) / (0.20\; \rm mol) = 8[/tex] hydrogen atoms.
The molecular formula of this hydrocarbon would be [tex]\rm C_{4} H_{8}[/tex].
You have 8 red M&Ms, 6 brown M&Ms, 4 yellow M&Ms, and 3 green M&Ms.
What percent of M&Ms are brown?
Answer to 1 Decimal Place (Check your rounding)
Answers
Answer:
the answer is : 28.6
1: 8+6+4+3 =21
2: there are 6 brown M&Ms = 6
3: %= 100
6×100÷21 = 28.57
round 1 Dec place = 28.6
The molecular formula mass of this compound is 120 amu . What are the subscripts in the actual molecular formula
Answers
Answer:
[tex]\mathbf{C_4 H_{8}O_{4}}[/tex]
Explanation:
From the given information:
Assume the molecular formula to be: [tex]C_a H_{2a} O_{a}[/tex]
Then, the molecular mass will be = 12a + 2a + 16a = 30a (since the C = 12, H = 2 & O = 16) respectively.
Thus;
30a = 120
a = 120/30
a = 4
Thus, the molecular formula:
[tex]=C_4 H_{2*4} O_{4}[/tex]
[tex]= \mathbf{C_4 H_{8}O_{4}}[/tex]
can u pls help me with this question
Answers
Answer:
D.
Explanation:
Well waves forming in not an indication of a phase change, neither is splashing water. Water soaking into the sand could be a form of change, from condensation into precipitation but that is not what the example is speaking of. The change from water into vapor can be indicated by the loss of water in liquid form.