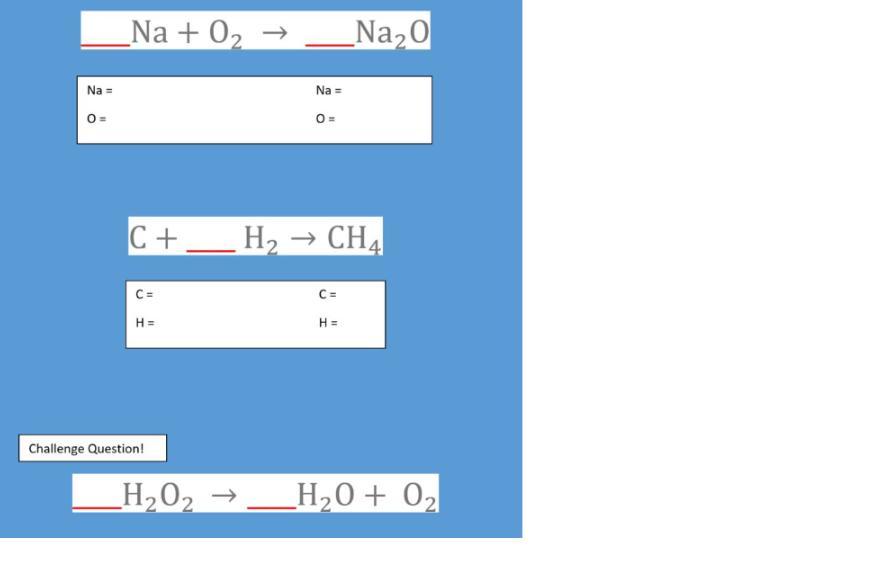
Balance the three equations below by adding the appropriate coefficients on the red lines.
In the text boxes below, count the number of atoms of each element on both sides of the reaction to make sure they are equal.
Answers
A balanced equation obeys the law of conservation of mass. The coefficients are the numbers that are added in front of the formulas to balance the equation.
What is a balanced equation?A balanced equation is defined as the chemical equation in which the mass of the products and reactants on either side of the equation will be equal. The number of atoms of each element on both sides of the equation are equal.
1. 4Na + O₂ → 2Na₂O
2. C + 2H₂ → CH₄
3. 2H₂O₂ → 2H₂O + O₂
Thus in the balanced equation, the number of atoms on both sides of the equation are equal.
To know more about balanced chemical equation, visit;
https://brainly.com/question/30702275
#SPJ1
Related Questions
What is molar mass of calcium phosphate?
Answers
The molar mass of calcium phosphate Ca₃(PO₄)₂ is 310 g. Molar mass is the aggregate of the masses of all the individual atoms present in a molecule or compound.
The chemical formula of calcium phosphate is Ca3(PO4)2. The atomic mass of calcium Ca is 40 u. The atomic mass of phosphorus P is 31 u. The atomic mass of Oxygen is 16 u.
The molecular mass of calcium phosphate = (atomic mass of calcium) × 3 + (atomic mass of phosphorus) × 2 + (atomic mass of oxygen) × 8
= (40 × 3) + (31 × 2) + (16 × 8)
= 310 g.
Therefore the molecular mass of calcium phosphate is 310 g.
Calcium phosphate, also called tricalcium phosphate, is a kind of mineral.
To learn more about the molar mass of calcium phosphate visit here:
https://brainly.com/question/24183428
#SPJ4
Copper has a delta. Hfus = 13. 0 kj/mol. What mass of copper releases 112. 4 kj of heat as it freezes?use q equals n delta h. 9. 42 g6. 75 g549 g1590 g.
Answers
The mass of copper releases 112. 4 kj of heat as it freezes be 549.4631 g.
How do you calculate the Heat of Fusion?Let Q be the Heat Required
n be the number of moles
number of moles = mass/molar mass
H be the Heat of Fusion = 13.0 kJ/mol
Let the equation be Q = n ΔH
n = Q / ΔH
substitute the values in the above equation, we get
n = 112.4 / 13
simplifying the equation, then
n = 8.65 mol
The number of moles of copper exists 8.65 mol.
To estimate the mass,
n = 8.65 mol
mass = 63.54 g/mol
m = M × n
substitute the values in the above equation, we get
= 8.65 mol × 63.54 g/mol
m = 549.4631 g
Therefore, the correct answer is option c) 549.4631 g.
To learn more about Heat of Fusion refer to:
https://brainly.com/question/87248
#SPJ4
which element has the lowest 1st ionization energy?
Answers
Bi is the element that has the lowest 1st ionization energy. Therefore, the correct option is option D.
The energy needed to ionise an atom or an ion in its gaseous form is equal to the energy needed to remove an electron from it. A positive ion is created when an atom loses one of its electrons, creating a species that is positively charged. The ionisation energy is represented in kilojoules per mole (kJ/mol) or electron volts (eV), which are common measures of energy per mole.
The five alternatives for elements are all members of the same group. As the group grows and the valence electrons are less tightly bonded, the value of ionisation energy (IE) decreases. Since Bi is the last element, it has the lowest IE in this scenario.
Therefore, the correct option is option D.
To know more about ionization energy, here:
https://brainly.com/question/33907239
#SPJ12
Your question is incomplete but most probably your full question was,
which element has the lowest 1st ionization energy?
A. As
B. P
C. N
D.Bi
E. Sb
which type of radioactivity has a negative charge?
A) alpha, α.
B) beta, β. C) gamma, γ. D) delta, δ
Answers
Answer:
Beta β
Explanation:
Beta particles are negatively charged electrons emitted by the nucleus on decay
what is nf3 compound name ?
Answers
The compound name for NF3 is nitrogen trifluoride.
NF3 is a compound that is commonly known as nitrogen trifluoride, is made up of one nitrogen atom and three fluorine atoms.
It is used in a variety of industrial processes, but it is also a potent greenhouse gas that contributes to global warming.
It is a colorless, odorless, and nonflammable gas that is used in the production of semiconductors, flat panel displays, and solar cells. It is also used as an etchant in the production of microelectronic devices.
Nitrogen trifluoride is a powerful greenhouse gas, with a global warming potential 17,200 times greater than that of carbon dioxide.
To know more about nitrogen trifluoride here:
https://brainly.com/question/9957071#
#SPJ11
All of the following statements are true about proteins except. -Proteins can have a structure characterized by loops, bend, and twists. -Proteins are composed of amino acids. -Proteins are polypeptides. Proteins are naturally acidic. -Hydrogen bonds keep the protein together
Answers
The statement that is not true about proteins is "Proteins are naturally acidic." Option C is correct.
Proteins are biological macromolecules made up of long chains of amino acids. They are also referred to as polypeptides because they are composed of multiple peptide bonds between the amino acids. The specific sequence and arrangement of amino acids in the polypeptide chain determine the unique 3D structure and function of the protein.
Proteins can have a structure characterized by loops, bends, and twists, and this structure is held together by a variety of intermolecular interactions, including hydrogen bonds, disulfide bonds, electrostatic interactions, and van der Waals forces.
While some amino acids have acidic or basic side chains, proteins as a whole are not necessarily naturally acidic. The overall charge of a protein is dependent on the charge of its constituent amino acids and the pH of the surrounding environment.
To know more about proteins here
https://brainly.com/question/30258984
#SPJ4
--The given question is incomplete, the complete question is
"All of the following statements are true about proteins except. -Proteins can have a structure characterized by loops, bend, and twists. A) Proteins are composed of amino acids. B) Proteins are polypeptides C) Proteins are naturally acidic. D) Hydrogen bonds keep the protein together"--
what is the boiling point elevation formula?
Answers
The formula for boiling point elevation is ΔTb = i×Kb×m. Where, i is the Van't Hoff factor, Kb is the ebullioscopic constant and m is the molality of the solute.
Generally, the boiling point elevation is defined as the difference in temperature that is created between the boiling point of the pure solvent and the boiling point of the solution. Basically, on the graph, the boiling point elevation is represented by ΔTb.
Basically, the molal boiling-point elevation constant is equal to the change in the boiling point for a 1-molal solution occurs in a nonvolatile molecular solute.
Molal elevation constant can be generally defined as the elevation in boiling point which is produced when one mole of non-volatile solute is dissolved in 1 kg i.e. 1000 g of the solvent. This constant is also known as the ebullioscopic constant. Hence, the unit of molal elevation constant is K Kg mol - 1 .
Learn more about boiling point elevation from the link given below.
https://brainly.com/question/16922336
#SPJ4
what is acetylcholine
Answers
Acetylcholine is a chemical messenger, or neurotransmitter, that is released by nerve cells in the brain and nervous system.
It plays an important role in the transmission of nerve impulses across the synapse, or gap, between nerve cells. Acetylcholine is involved in many functions of the body, including muscle movement, memory, and cognition.
Acetylcholine is released from the presynaptic neuron into the synapse, where it binds to receptors on the postsynaptic neuron. This binding triggers an electrical signal, or action potential, in the postsynaptic neuron, which then propagates the nerve impulse to other cells.
After it has done its job, acetylcholine is broken down by an enzyme called acetylcholinesterase, so that it can no longer bind to the receptors and trigger an action potential.
Acetylcholine is an important neurotransmitter in both the central nervous system (CNS) and the peripheral nervous system (PNS). In the CNS, it is involved in memory and cognition. In the PNS, it is involved in the control of muscle movement, including the contraction of skeletal muscles and the regulation of smooth muscles in the digestive tract, lungs, and other organs.
To know more about Acetylcholine here:
https://brainly.com/question/29855206#
#SPJ11
What happens when sulphide ore is roasted?
Answers
When sulphide ore is roasted the sulphide ores generally gets converted to their respective oxides and the impurities are also oxidised and removed.
Generally roasting is defined as the process in which the mainly sulphide ores are heated below their melting point in presence of air. The sulphide ores are converted to their respective oxides and the impurities are also oxidized and removed.
During the process of roasting, the ore or the ore concentrate is treated with very hot air. Roasting is the process that is generally applied to sulfide minerals. During the process of roasting, the sulfide ore is usually converted to an oxide, and sulfur is released as sulfur dioxide, a gas.
Learn more about roasting from the link given below.
https://brainly.com/question/29280192
#SPJ4
Which of the following is NOT a possible pair of quantum numbers?
Multiple Choice:
2p
2d
4p
4f
Answers
The 2d orbital shell where n = 2 , has not a possible pair of quantum numbers. So, correct choice is option (b).
Quantum numbers specify the properties of atomic orbitals and the electrons in those orbitals. Each electron in an atom is described by a set of four numbers:
Principal quantum number (n)Angular momentum quantum number (l)Magnetic quantum number (ml)Spin quantum number (ms)The spin quantum number indicates the two possible orientations of the electron's spin. The value of the spin quantum number can never be zero because electrons always have either positive or negative spin. The rules for quantum numbers are:
'n' can be any positive non-zero integral value.'l' can be zero or any positive integer but not greater than (n-1). l = 0, 1, 2, 3, 4, ….(n-1) 'mₗ', the values correspond to Eq. -l, +1, +2, +3, +l'mₛ', can be +1/2 or -1/2.Now check the options for 2p ; n = 2, l = 1, mₗ = -1,0,1, this set of quantum numbers is possible.
For 4p, n = 4, l = 1, mₗ = -1, 0, 1. So it's a possible orbital.
For 2d, n = 2, l = 2 (for d orbital), but according to the rule, the value of l cannot equal n, so a 2d orbital is not possible.
For 4f, n = 4, l = 3, mₗ = -3,-2,-1,0,1,2,3. So it is possible. So, a 2d orbital is not possible.
For more information about quantum numbers, refer:
https://brainly.com/question/24095340
#SPJ4
What is the acetic acid constanta (ka)?
Answers
The value of Ka constant for acetic acid is 1.75 × 10⁻⁵.
Generally, the acid dissociation constant (Ka) is used to distinguish strong acids from weak acids. Strong acids generally have exceptionally high Ka values. The Ka value is obtained by looking at the equilibrium constant for the dissociation of the acid. The higher is the Ka, the more the acid dissociates into its ions.
Ka is defined as the acid dissociation constant whereas pKa is simply the -log of the constant Ka. Similarly, Kb is defined as the base dissociation constant, whereas pKb is the -log of the constant Kb.
Learn more about dissociation constant from the link given below.
https://brainly.com/question/28197409
#SPJ4
Find the acceleration rate if the force is 200 N and the mass of an object is 150 kg.
Answers
Answer:
Explanation:
F=ma
200=150*a
a=5/4=1.25m/s^2
Explain how the concentration of a solute in a solution influences its boiling point and freezing point.
PLEASE BE ACCURATE!!! Thank you!!:))
Answers
The addition of a non-volatile solute into a solvent decreases its freezing point and increases its boiling point. Both these changes are colligative property. As the solute concentration increases, the changes also increases.
What are colligative properties ?Colligative properties are those properties which depends on the amount of the substance. For example elevation of boiling point is a colligative property which increases with the concentration of the solute added.
When a non -volatile solute is added to the solvent, the solvent -solvent bonds becomes weaken and solvent -solute bonds make the solvent molecules difficult to escape into vapor phase. This will increase the boiling point.
Similarly, the addition of salt makes the solvent molecules in intact and the intermolecular force between solute-solvent molecules makes them easily freeze which leads to depression in freezing point.
Both these changes are colligative property. As the concentration of solute increases, the elevation of boiling point or the depression in freezing point increases.
Find more on colligative property:
https://brainly.com/question/10323760
#SPJ1
What type of intramolecular force is the bond between Carbon and Oxygen in CO2? A. ionic bonds
B.polar covalent bonds
C.nonpolar covalent bonds
D.metallic bonds
Answers
The type of intramolecular force is the bond between Carbon and Oxygen in CO₂ is polar covalent bond.
A polar covalent bond is defined as a type of covalent bond in which the atoms have an unequal attraction for electrons, and so the sharing is unequal. In a polar covalent bond, which is sometimes also called as a polar bond, the distribution of electrons around the molecule is no longer symmetrical.
The type of bond present between the atoms in a molecule of CO₂ is polar covalent bond. In a molecule of carbon dioxide, a carbon atom is joined by four covalent bonds to two oxygen atoms, which have two covalent bonds each.
Learn more about polar covalent from the link given below.
https://brainly.com/question/10777799
#SPJ4
Based on the trends on the periodic table, predict what the reaction between rubidium and water and cesium and water will be. How will they be similar to sodium and potassium in water and how will they be different. Explain why you made this prediction
Answers
Sodium and potassium will react with water to form hydroxides with chemical formula as,NaOH and KOH ,and RuOH.
What is chemical formula?Chemical formula is a way of representing the number of atoms present in a compound or molecule.It is written with the help of symbols of elements. It also makes use of brackets and subscripts.
Subscripts are used to denote number of atoms of each element and brackets indicate presence of group of atoms. Chemical formula does not contain words. Chemical formula in the simplest form is called empirical formula.
Learn more about chemical formula,here:
https://brainly.com/question/29031056
#SPJ1
Use the reaction equation and bond energies to answer the question.
2H₂O 2H₂ + O₂
H-O: 467 kJ/mol
H-H: 432 kJ/mol
-
O=O: 498 kJ/mol
What is the total energy of the reaction? Is this an endothermic or exothermic reaction?
(1 point)
O -506 kJ/mol, exothermic
O-506 kJ/mol, endothermic
O 506 kJ/mol, endothermic
O 506 kJ/mol, exothermic"
Answers
To determine the total energy of the reaction, we need to calculate the energy required to break the bonds in the reactants and the energy released when new bonds are formed in the products.
How to determine total energy ?In the reactants, we have two H-O bonds and one O=O bond:2H-O: 2 x 467 kJ/mol = 934 kJ/mol
1O=O: 1 x 498 kJ/mol = 498 kJ/mol
Total energy required to break bonds in the reactants: 1432 kJ/molIn the products, we have four H-H bonds and one O=O bond:
4H-H: 4 x 432 kJ/mol = 1728 kJ/mol
1O=O: 1 x 498 kJ/mol = 498 kJ/mol
Total energy released when new bonds are formed in the products: 2226 kJ/molTo find the total energy of the reaction, we need to subtract the energy required to break bonds in the reactants from the energy released when new bonds are formed in the products:Total energy of the reaction = energy released - energy required = 2226 kJ/mol - 1432 kJ/mol = 794 kJ/mol
To know more about total energy , check out :
https://brainly.com/question/14427111
#SPJ1
What do you mean by the law of mass action?
Answers
The law of mass action is a chemical principle that states that the rate of a chemical reaction is directly proportional to the product of the concentrations of the reactants.
This means that the more reactants you have, the faster the reaction will proceed.
The law of mass action can be expressed mathematically as:
rate = k[A][B]
where k is the rate constant, [A] and [B] are the concentrations of the reactants, and the rate is the speed at which the reaction proceeds.
The law of mass action is important in understanding how chemical reactions work and how to control them. By manipulating the concentrations of the reactants, we can control the rate of the reaction and therefore control the outcome of the reaction.
In summary, the law of mass action is a fundamental principle in chemistry that describes how the rate of a chemical reaction is affected by the concentrations of the reactants.
To know more about law of mass action here:
https://brainly.com/question/951024#
#SPJ11
scientist can use ____________ to measure evidence that electrons can move from one energy level to another.
Answers
Scientists can use spectroscopy to measure evidence that electrons can move from one energy level to another. Scientists can measure the energy added when electrons absorb energy and move to higher energy levels.
ExplanationSpectroscopy is a technique that involves shining light on a material and measuring how that material absorbs or emits light at different wavelengths. When electrons move from one energy level to another, they can absorb or emit photons of light, which results in a characteristic spectral signature.
For example, in atomic spectroscopy, scientists can measure the absorption or emission of light by atoms when electrons jump between different energy levels. This provides valuable information about the electronic structure of atoms and molecules, including the energy levels of electrons and the bonding between atoms.Learn more about spectroscopy on:
brainly.com/question/14854785https://brainly.com/question/28457917https://brainly.com/question/22509226When the free energy of the reaction is lower in the products than it is in the reactant the reaction is?
Answers
When the free energy of the reaction is lower in the products than it is in the reactants, the reaction is exergonic.
An exergonic reaction is a spontaneous chemical reaction that releases energy as a result of the products having lower free energy than the reactants. This means that the reaction can occur spontaneously without the need for additional energy input. The difference in free energy between the reactants and the products determines the amount of energy that is released during the reaction. In contrast, an endergonic reaction is a non-spontaneous chemical reaction that requires energy input to occur, and the free energy of the products is higher than that of the reactants.
You can learn more about exergonic reaction at
https://brainly.com/question/1560981
#SPJ4
If a 1.0 L canister holds 2.0 moles of gas, and the temperature of the gas is 500. C, what is the pressure inside the container in atm
Answers
The pressure of the gas inside the canister can be determined using the ideal gas equation. The pressure inside the canister is 126.7 atm.
What is ideal gas equation ?Ideal gas equations states the relation between volume, pressure, temperature and number of moles of gas as written below:
PV = nRT.
Where R is the universal gas constant equal to 0.082 L atm/K mol.
Given the volume of the tank V = 1 L
number of moles n = 2 moles
temperature = 500 ° C = 773 k
Then pressure P = nRT/V.
Pressure of gas = 2 moles ×773 K× 0.082 L atm/K mol /(1 L)
P = 126 atm.
Therefore, the pressure of the gas in the canister is 126 atm.
Find more on ideal gases:
brainly.com/question/30247106
#SPJ9
what is the vsepr geometry is most likely for the carbon atom in this transition state?
Answers
The VSEPR geometry for the carbon atom in the transition state would depend on the specific molecule and reaction being considered.
VSEPR, or Valence Shell Electron Pair Repulsion, the theory is used to predict the shape of molecules based on the distribution of electron pairs around the central atom. In a transition state, the molecule is in a high-energy, intermediate stage of a chemical reaction, and its shape may be different from the starting materials or final products.
The VSEPR geometry of the carbon atom in the transition state would depend on the specifics of the molecule and reaction, including the number of electron pairs around the carbon atom and the types of atoms bonded to it. To determine the VSEPR geometry for a specific transition state, it is necessary to have detailed information about the molecule and reaction in question.
Learn more about VSEPR geometry:
https://brainly.com/question/14225705
#SPJ4
Interconverting molar mass and density of ideal gases Calculate to three significant digits the density of dinitrogen monoxide gas at exactly 20 °C and exactly 1 atm.You can assume dinitrogen monoxide gas behaves as an ideal gas under these conditions.
Answers
The density of dinitrogen monoxide gas at exactly 20 °C and exactly 1 atm is 1.83 g/L.
The problem states that we can treat this gas as an ideal gas, therefore, we can use the equation of an ideal gas which is:
PV = nRT
Now, the density (d) is calculated as:
d = m/V
We can rewrite above equation,
m = d*V
Now, the moles (n),
n = m /MM
The ideal gas equation is,
d = P * MM / RT
The molar mass of N₂O is 44 g/mol.
So, replacing all the data we have:
[tex]d = \dfrac{1 \times 44}{0.082 \times 293}[/tex]
d = 1.83 g/L
To know more about the ideal gas equation, here
brainly.com/question/28837405
#SPJ4
what is zinc's atomic number?
Answers
The atomic number of zinc is 30.
Generally, the atomic number is simply defined as the number of protons present in an atom. Due to this reason, atomic number is also sometimes known as the proton number. During the calculations, atomic number is usually denoted by the capital letter Z. The symbol Z is derived from the a German word zahl, which means number of numeral, or atomzahl, which is a more modern word which means atomic number.
The atomic number is basically the number of protons present in the nucleus of an atom. The number of protons are used to define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present in the atom, identity depends upon the number of protons.)
Learn more about atomic number from the link given below.
https://brainly.com/question/16858932
#SPJ4
Mg + 2HCl yields MgCl2 + H2 If 4. 00 g of Mg is reacted with 3. 20 g of HCl How many grams of magnesium Chloride is produced in the reaction?
Answers
The mass of magnesium chloride produced in the reaction is 4.17 grams.
The balanced chemical equation for the reaction between Mg and HCl is:
Mg + 2HCl → MgCl2 + H2
To solve this problem, we need to determine which reactant is limiting and use its stoichiometry to calculate the amount of product produced.
First, we need to find the number of moles of Mg and HCl used in the reaction:
moles of Mg = 4.00 g / 24.31 g/mol = 0.1643 mol
moles of HCl = 3.20 g / 36.46 g/mol = 0.0878 mol
According to the balanced equation, the reaction uses 1 mole of Mg for every 2 moles of HCl. Therefore, Mg is in excess and HCl is the limiting reagent.
The stoichiometry of the balanced equation tells us that 2 moles of HCl produce 1 mole of MgCl2. So, the number of moles of MgCl2 produced can be calculated as:
moles of MgCl2 = 0.0878 mol HCl × (1 mol MgCl2 / 2 mol HCl) = 0.0439 mol MgCl2
Finally, we can calculate the mass of MgCl2 produced using its molar mass:
mass of MgCl2 = 0.0439 mol × 95.21 g/mol = 4.17 g
Therefore, the mass of magnesium chloride is 4.17 grams.
To know more about mass:
https://brainly.com/question/15199003
#SPJ4
What happens when you titrate a weak acid with a strong base?
Answers
When we titrate a weak acid with a strong base, there is a sharp increase in pH at the beginning of the titration.
During the titration of a weak acid with a strong base, very often there is a sharp increase in pH at the beginning of the titration. This happens because the anion of the weak acid becomes a common ion which reduces the ionization of the acid. Soon after the sharp increase at the beginning of the titration the curve only changes gradually. This is caused because the solution is only acting as a buffer.
General titration of an acid with a strong base:
Basically, at equivalence point, the only species which is present in the solution are the neutral ions (the cation from the strong base and the anion from the strong acid) and water.
Learn more about titration from the link given below.
https://brainly.com/question/2728613
#SPJ4
what is the bond order of n2?
Answers
The bond order of N₂ is three.
Generally, bond order is described as the number of bonding pairs of electrons that is present between two atoms. Basically, between two atoms in a covalent bond, the bond order of a single bond is of one, the bond order of a double bond is of two, and the bond order of a triple bond is of three, and trend goes so on.
Mathematically,
Bond order = [Bonding molecules' number of electrons – Antibonding molecules' number of electrons] × 1/2.
Hence, bond order of both N2+ and N2- is 3, but according to the molecular orbital theory, N2- has more antibonding electrons than N2+.
Learn more about bond order from the link given below.
https://brainly.com/question/12447843
#SPJ4
determine whether each of the examples represents a colligative property or a non-colligative property.
Answers
Examples of colligative properties include boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure.
Examples of non-colligative properties include color, density, and refractive index. These properties depend on the identity of the solute particles and not on their concentration.
Colligative properties are properties of a solution that depend on the concentration of solute particles in the solution and are independent of the identity of the solute particles. Non-colligative properties, on the other hand, are properties of a solution that are dependent on the identity of the solute particles.
It is important to note that while colligative properties are not affected by the identity of the solute particles, they do depend on the number of particles in the solution. Therefore, colligative properties can be used to determine the molecular weight of a solute or the degree of dissociation of an ionic compound.
Find out more about colligative property or a non-colligative property
brainly.com/question/29971068
#SPJ4
Review the reversible reactions given, along with the associated equilibrium constant K at room temperature. In each case, determine whether the forward or reverse reaction is favored.CH3COOH â CH3COO- + H+ Ka=1.8 x 10-5 choose...reverse or forwardA + B â C K=4.9 x 103 choose... reverse or forwardAgCl â Ag+ + Cl- Ksp=1.6 x 10-10 Choose... reverse or forwardAl(OH)3 â Al3+ + 3OH- Ksp=3.7 x 10-15 Choose... reverse or forward
Answers
The forward reaction rate constant divided by the reverse reaction rate constant is equal to the equilibrium constant.
What is an equilibrium?Chemical equilibrium in a reaction is the situation in which both the reactants and products are present at concentrations that do not continue to fluctuate over time, preventing any discernible change in the system's features. During a reversible chemical reaction, the situation known as chemical equilibrium occurs when there is no net change in the quantity of reactants or products.is a situation where the rate of the forward reaction is equal to the rate of the backward reaction. The definition of OR includes the phrase "A condition where the concentration of reactant and product remains constant." A good example is H2(g)+I2(g)HI (g)When a response shifts to equilibrium, its direction can be predicted using Q.To learn more about equilibrium refer to:
https://brainly.com/question/18849238
#SPJ4
Arrange these compounds by their expected boiling point.a. CH3OH b. CH4 c. CH3Cl
Answers
The expected boiling points of the compounds, in order from highest to lowest, would be CH3OH > CH3Cl > CH4
CH3OH - Methanol is a polar organic compound with a boiling point of 64.7°C.
CH4 - Methane is a non-polar organic compound with a boiling point of -164°C.
CH3Cl - Chloromethane is a polar organic compound with a boiling point of 40.1°C.
Therefore, the expected boiling point order is CH4 < CH3Cl < CH3OH.
The temperature at which a material transforms from a liquid into a gas is known as its boiling point.This is usually determined by the vapor pressure of the substance; when the vapor pressure of a liquid is equal to the atmospheric pressure, it boils and changes to a gas.
learn more about boiling point Refer:brainly.com/question/2153588
#SPJ4
Pushing
13N
Pulling
16N
Net Force =