Answers
Answer:
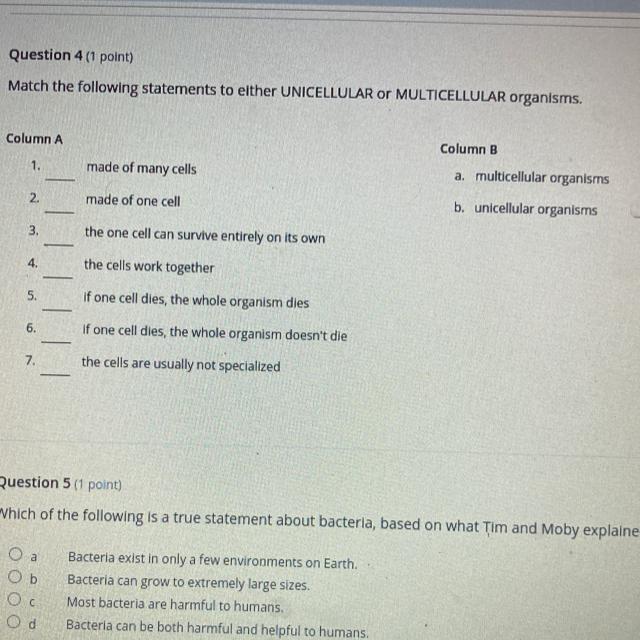
qns-4
1-multicellular organism
2-unicellular organism
3-unicellular organism
4-multicellular organism
5-unicellular organism
6-multicellular organism
7-unicellular organism
Related Questions
Describe a time in the group discussion when you referred to the notes you took, the graphic organizer you filled out, and your position paper.
Answers
Answer:
To back up any statements I make.
To refute any errors presented.
Explanation:
During a group discussion, you look at your notes to point out an error or when you want to flesh out a point you made.
What is a group discussion?The term group discussion has to do with learners talking about a topic in a manner ion which each learner takes a turn to explain a portion of the topic.
During a group discussion, you look at your notes to point out an error or when you want to flesh out a point you made.
Learn more about group discussion:https://brainly.com/question/11940982
#SPJ2
Carbon is more stable element than Silicon. True or False?
Answers
Which compounds will most likely dissociate when dissolved in water? Select all that apply.
Answers
Answer:
A) barium hydroxide
B) ethanol
C) glucose
D) silver nitrate
E) dichloromethane
F) postassium chloride
The other compounds ethanol and glucose are also soluble in water but do not dissociate when dissolved in water.
Explanation:
Please mark this Brainilest.
These compounds will dissociate in water:
A) barium hydroxide
D) silver nitrate
F) potassium chloride
What are ionic compounds?Many compounds do separate when they are dissolved in water. Ionic substances break apart into a positive ion and a negative ion when placed in water, a process known as electrolytic or ionic dissociation. Ionization is the name given to this kind of dissociation reaction.When dissolved in water, many substances do dissociate. It is claimed that substances that dissolve in water form electrolytes, which are ions.Electrolytes can disintegrate into cations and anions after being dissolved, or they may be ionic substances that chemically react with water to produce ions.learn more about dissociation here: https://brainly.com/question/305470
#SPJ2
what kind of chemical reaction is this?
Answers
Plz help! I will give brainliest.
Answers
Answer:
D. 0.50
Explanation:
Use avogadro number to find the whole work.
Name the following compound:
CH2 ≡ CH2
Ethyl
Ethyne
Ethane
ethane
Answers
Answer:
Answer is A. Ethyl.........
Answer:
D. ethane
Explanation:
NH₄NO₃ → N₂O + 2H₂O When 45.70 g of NH₄NO₃ decomposes, what mass of each product is formed?
Answers
Answer: 25.13 g of [tex]N_2O[/tex] and 20.56 g of [tex]H_2O[/tex] will be produced from 45.70 g of [tex]NH_4NO_3[/tex]
Explanation:
To calculate the moles :
[tex]\text{Moles of solute}=\frac{\text{given mass}}{\text{Molar Mass}}[/tex]
[tex]\text{Moles of} NH_4NO_3=\frac{45.70g}{80.04g/mol}=0.571moles[/tex]
The balanced chemical equation is:
[tex]NH_4NO_3\rightarrow N_2O+2H_2O[/tex]
According to stoichiometry :
1 mole of [tex]NH_4NO_3[/tex] produce = 1 mole of [tex]N_2O[/tex]
Thus 0.571 moles of [tex]NH_4NO_3[/tex] will require=[tex]\frac{1}{1}\times 0.571=0.571moles[/tex] of [tex]N_2O[/tex]
Mass of [tex]N_2O=moles\times {\text {Molar mass}}=0.571moles\times 44.01g/mol=25.13g[/tex]
1 mole of [tex]NH_4NO_3[/tex] produce = 2 moles of [tex]H_2O[/tex]
Thus 0.571 moles of [tex]NH_4NO_3[/tex] will require=[tex]\frac{2}{1}\times 0.571=1.142moles[/tex] of [tex]H_2O[/tex]
Mass of [tex]H_2O=moles\times {\text {Molar mass}}=1.142moles\times 18g/mol=20.56g[/tex]
Thus 25.13 g of [tex]N_2O[/tex] and 20.56 g of [tex]H_2O[/tex] will be produced from 45.70 g of [tex]NH_4NO_3[/tex]
is butanoic acid a hydrocarbon?
Answers
Yes butanoic acid is a hydrocarbon.
HELPPP ASAPPP
Use the element tile below to calculate the molar mass of He2 (helium gas).
2 g/mol
16.012 g/mol
8.006 g/mol
4.003 g/mol
Answers
Answer: 4.003 g/mol
Explanation:
So basically Molar mass is the atomic mass of an element and the atomic mass of Helium is 4.0026 or 4.003 g/mol
multiply the molar mass of helium (4.003) by the subscript and you have your answer!
Determine the percent yield for the reaction between 46.5 g of
ZnS and 13.3 g of oxygen if 18.l4 g of ZnO is recovered along with an unknown quantity of sulfur
dioxide.
please show work thank you
Answers
The percentage yield of the reaction has been 46.74%.
Percentage yield can be given as the ratio of the actual yield to the theoretical yield.
For the theoretical yield, the balanced equation will be:
[tex]\rm 2\;ZnS\;+\;3\;O_2\;\rightarrow\;2\;ZnO\;+\;2\;SO_2[/tex]
Thus, 2 moles of ZnS will give 2 moles of ZnO.
The moles of 46.5 g ZnS:
Moles = [tex]\rm \dfrac{weight}{molecular\;weight}[/tex]
ZnS = [tex]\rm \dfrac{46.5}{97.474}[/tex]
ZnS = 0.477 mol
The moles of 13.3 grams Oxygen:
Oxygen = [tex]\rm \dfrac{13.3}{32}[/tex]
Oxygen = 0.415 mol.
The moles of 18.14 grams ZnO:
ZnO = [tex]\rm \dfrac{18.14}{81.38}[/tex]
ZnO = 0.222 mol
According to the balanced chemical equation,
2 moles ZnO = 2 moles ZnS
0.222 mol ZnO = 0.222 mol ZnS
2 moles ZnO = 3 moles Oxygen
0.222 mol ZnO = 0.334 mol of Oxygen
Since, both the reactants are in enough concentration,
The theoretical yield can be calculated with any of the reactants. The theoretical yield of ZnO from 46.5 grams of ZnS can be given as:
1 mole ZnS = 1 mol ZnO
0.477 mol of ZnS = 0.477 mol of ZnO.
The mass of 0.477 mol of ZnO:
Mass = moles [tex]\times[/tex] molecular weight
Mass of ZnO = 0.477 [tex]\times[/tex] 81.38
Mass of ZnO = 38.818 grams.
The theoretical yield of ZnO = 38.818 g.
The actual yield of ZnO = 18.14 g.
Percentage yield = [tex]\rm \dfrac{18.14}{38.81}\;\times\;100[/tex]
Percentage yield = 46.74 %
The percentage yield of the reaction has been 46.74%.
For more information about the percentage yield, refer to the link:
https://brainly.com/question/11715808
Can Intrusive and Extrusive Igneous rocks form from the same mineral composition?
Answers
EXAMPLE took the test k12
A pebble is dropped into a cup of water and sinks to the bottom ofthe cup. A solid metal bead of exactly the same size is dropped into the same cup and sinks to the bottom of the cup. How do the pebble and the metal bead compare?
Answers
Answer:
A solid metal bead of the same size is dropped into the same cup and sinks to the bottom of the cup. How do the pebble and the metal bead compare? They both have the same mass. They are both denser than water.
Explanation:
nun
Answer:
Both the pebble and metal bead of the same mass will be equally accelerated if they are at the same place, in this case, they are in the same place meaning they will be accelerated at the same speed towards the earth. Thus even if they are in the water they will sink at the same speed and finally will reach the bottom of the cup.
How many particles are in one mole of copper (II) sulfate, CuSO4?
Answers
When measuring the brightness of a star, what is a limitation of using apparent magnitude rather than absolute brightness?
Apparent magnitude does not use the star's color or temperature.
Apparent magnitude is only theoretical, thus its value cannot be directly measured with equipment.
Apparent magnitude does not take into account the fact that not all stars are the same distance from Earth.
Apparent magnitude is only on a comparative scale that determines how bright a star looks from a theoretical distance of 32 light-years from Earth.
Answers
Answer: Answer:
Explanation:
Apparent magnitude does not take into account the fact that not all stars are the same distance from Earth.
Apparent magnitude is only on a comparative scale that determines how bright a star looks from a theoretical distance of 32 light-years from Earth. Therefore, the correct option is option D.
What is star?Any large, gaseous celestial body that is capable of producing its own light and illuminates through radiation. Only a very small portion of the tens of milliards of milliards of stars that make up the observable cosmos are readily visible to the eye.
When measuring the brightness of a star, what is a limitation of using apparent magnitude rather than absolute brightness. Apparent magnitude is only on a comparative scale that determines how bright a star looks from a theoretical distance of 32 light-years from Earth.
Therefore, the correct option is option D.
To know more about star, here:
https://brainly.com/question/18426562
#SPJ6
The Earth has how many satellites?
(i know this is not chemistry theres no science one)
Answers
Answer:
Well it depends, There are THOUSANDS of inactive satellites. Even scientists don't know how many as for active ones there is about 1 957 according to nasa
Explanation:
What is the pOH if the [OH-]= 0.165 M? What is the pH of this basic solution? *Please round your answer(s) to the appropriate number of significant figures. Your answer can be in standard notation or i letter "e" in place of x10.* 1 N
Answers
Answer:
78
Explanation:
Answer:
The OH would be .7825 and the basic solution is a strong base.
Explanation:
What you would do is -log(0.165) in your calculator which would give you 0.7825160065 as an answer. Im not sure what the significant figure is so I will not be rounding to that, but that is your answer for the first part.
The second part: because your pOH is a .7825, this would be consiered a strong base in the pOH because it is closer to 1 which is your base.
The kinetic molecular theory, explains how particles in matter behave.
True
False
Answers
Answer:
true
Explanation:it is hard to explan
What happens when dilute sulphuric acid is poured on a copper plate?
Answers
PLEASE HELP ME QUICKLY!
Imagine you are at your favorite beach. The sun is shining and you are enjoying the ocean breeze. The temperature is about 89F. You take your shoes off and realize that the sand is almost too hot to walk on. You run to the water's edge to wet your feet after the walk over the sand and realize that the water is almost too cold. Explain the temperature difference between the sand and water using Thermodynamics.
Answers
Answer:
The specific heat of water is more specific than the heat of sand, therefore, it will take more energy to raise the same amount of water with the same temperature.
Explanation:
The temp is 89 F so, 31.67° C
The specific heat of the water is 4.184 J/g° C
The specific heat of the sand is 0.290 J/ g° C
The heat is the amount of energy that is needed to raise the temp. of the substance
You will need a lot more energy to raise the temp of the water then of the same amount of sand, therefore, because of the specific lower heat of the sand it will raise it's temperature quicker compared to water.
Please someone help me ...............
Answers
Answer:
Q58. number of moles(n) and relative formula mass(Mr)
Q59. a) 2 H2S + O2 --> 2 H2O + 2 S
b) After the balanced equation you should able to draw it yourself - I cannot draw it on here :)
Explanation:
Which statement best describes DNA?
(A) It can be found in every living cell.
B It can be found only in specialized
cells.
© It can be found only in cells with a
nucleus.
D It can be found only when cells are
dividing.
Answers
Answer:
Found in every living cell
The force that is pulling down on a person or object on the surface of the Earth is
called (attraction of matter to matter)?
normal force
mass
weight
gravity
Answers
Have a good day/night
Molar Volume of a gas at STP=22.4 L Example 3: Determine the volume of Carbon dioxide created when 15.0 grams of NaHCO3 are decomposed into water, Sodium carbonate, and Carbon dioxide at STP?
Answers
Answer:
20009 is answer is right 1345678899444
Explanation:
thhjbfthvcdthvctyhgffdy
gvhjk ghkde ghjcddxxb hhj hhgddxb ggjbcxdss ggbbsrtyg fy gbsdgh
How many grams are there in 9.03 x 1023 molecules of CO2?
Answers
Answer:
The correct answer is - 66g.
Explanation:
Given:
molecules of CO2 = 9.03 x 10^23
We know:
1 mole of any substance = 6.02x10^23 molecules. (Avogadro's number)
M (CO2) = 12 + (2x16) = 12 + 32 = 44g
Solution:
The mass of CO2 with 9.03x10^23 molecules would be:
44g of CO2 = 6.02x10^23 molecules.
So, the mass in grams of CO2 (X) = 9.03x10^23 molecules
Xg of CO2 = (44x9.03x10^23)/6.02x10^23 = 66g
Thus, the correct answer would be - 66gm.
2.50 L of gas originally at 50 K is warmed to 80 K. If the pressure remains constant, which gas law needs to be used to find the new volume?
Select one:
a.Charles law
b.Gay-Lussac’s law
c.Boyle’s law
d.Combined gas law
Answers
In the following reaction 2Na+ 2H20 - 2NaOH + H2: all of the sodium is consumed and a small amount of
liquid water remains at the end of the reaction. Which reactant is the limiting reagent?
A) H20
B) H2
C) Na
D) NaOH
Answers
Answer:
C) Na
Explanation:
The question tells us that all of the sodium (Na) is consumed and that water is left over. This means that sodium would be the limiting reagent.
I took the test
The formula for zirconium(VI) dichromate
Answers
Answer:
Zr(Cr2O7)2
Explanation:
1. The pressure of a gas is 100.0 kPa and its volume is 500.0 ml. If the volume increases to 1,000.0 ml, what is the new pressure of the gas?
2. If a gas at 25.0 °C occupies 3.60 liters at a pressure of 10 kPa, what will be its volume at a pressure of 25 kPa?
3. When the pressure on a gas increases three times, by how much will the volume increase or decrease?
4. Boyle's Law deals what quantities?
Answers
Answer:
1) The new pressure of the gas is 500 kilopascals.
2) The final volume is 1.44 liters.
3) Volume will decrease by approximately 67 %.
4) The Boyle's Laws deals with pressures and volumes.
Explanation:
1) From the Equation of State for Ideal Gases we construct the following relationship:
[tex]\frac{P_{2}}{P_{1}} = \frac{V_{1}}{V_{2}}[/tex] (1)
Where:
[tex]P_{1}, P_{2}[/tex] - Initial and final pressure, measured in kPa.
[tex]V_{1}, V_{2}[/tex] - Initial and final pressure, measured in mililiters.
If we know that [tex]P_{1} = 100\,kPa[/tex], [tex]V_{1} = 500\,mL[/tex] and [tex]V_{2} = 1000\,mL[/tex], then the new pressure of the gas is:
[tex]P_{2} = P_{1}\cdot \left(\frac{V_{1}}{V_{2}} \right)[/tex]
[tex]P_{2} = 500\,kPa[/tex]
The new pressure of the gas is 500 kilopascals.
2) Let suppose that gas experiments an isothermal process. From the Equation of State for Ideal Gases we construct the following relationship:
[tex]\frac{P_{2}}{P_{1}} = \frac{V_{1}}{V_{2}}[/tex] (1)
Where:
[tex]P_{1}, P_{2}[/tex] - Initial and final pressure, measured in kPa.
[tex]V_{1}, V_{2}[/tex] - Initial and final pressure, measured in mililiters.
If we know that [tex]V_{1} = 3.60\,L[/tex], [tex]P_{1} = 10\,kPa[/tex] and [tex]P_{2} = 25\,kPa[/tex] then the new volume of the gas is:
[tex]V_{2} = V_{1}\cdot \left(\frac{P_{1}}{P_{2}} \right)[/tex]
[tex]V_{2} = 1.44\,L[/tex]
The final volume is 1.44 liters.
3) From the Equation of State for Ideal Gases we construct the following relationship:
[tex]\frac{P_{2}}{P_{1}} = \frac{V_{1}}{V_{2}}[/tex] (1)
Where:
[tex]P_{1}, P_{2}[/tex] - Initial and final pressure, measured in kPa.
[tex]V_{1}, V_{2}[/tex] - Initial and final pressure, measured in mililiters.
If we know that [tex]\frac{P_{2}}{P_{1}} = 3[/tex], then the volume ratio is:
[tex]\frac{V_{1}}{V_{2}} = 3[/tex]
[tex]\frac{V_{2}}{V_{1}} = \frac{1}{3}[/tex]
Volume will decrease by approximately 67 %.
4) The Boyle's Laws deals with pressures and volumes.
Increasing the amount of current flowing through a wire strengthens
what?
magnetic field
electromagnetism
solenoid
O polarity
Answers
Answer:
It will strengthen Electromagnetism
3. Identify the 4 primary pigments found in plant leaves (name and color)
Answers
Anthocyanins: red
Carotene: orange.
Sorry I couldn’t find the 4th one