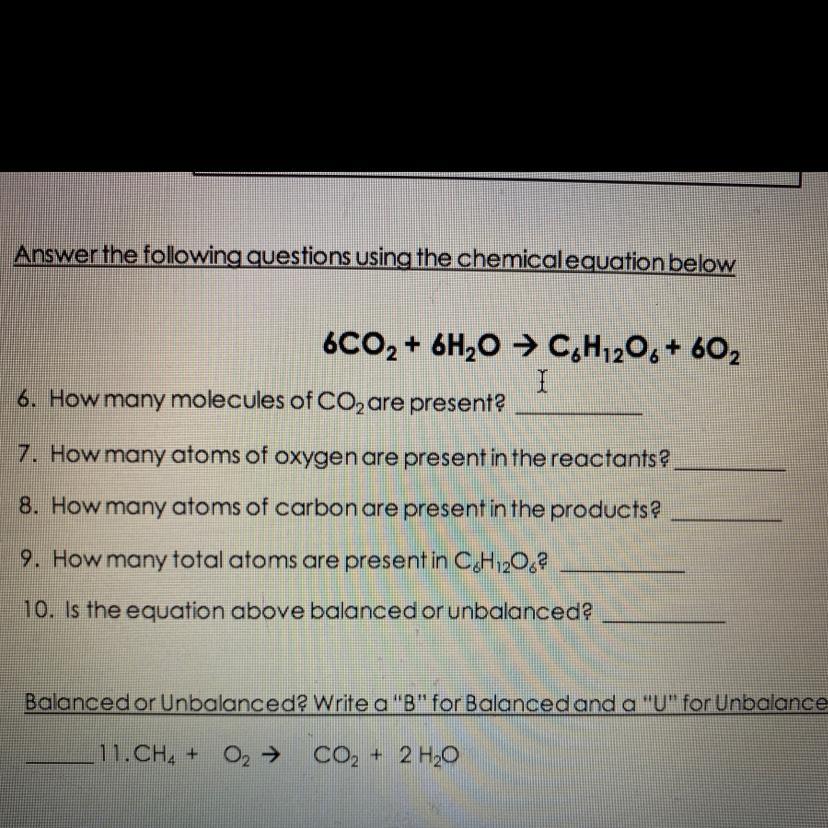
6CO2 + 6H20 → C,H120, + 602
6. How many molecules of CO2 are presenta
7. How many atoms of oxygen are present in the reactants?
8. How many atoms of carbon are present in the products
9. How many total atoms are present in C.H120,?
10. Is the equation above balanced or unbalanced?
Answers
Explanation:
The equation of the reaction is given as;
6CO2 + 6H2O → C6H12O6 + 6O2
How many molecules of CO2 are present?
6 moles
How many atoms of oxygen are present in the reactants?
(6 * 2) + (6 * 1 ) = 12 + 6 = 18
How many atoms of carbon are present in the products?
(6 * 1 ) = 6
How many total atoms are present in C6H12O6?
6 + 12 + 6 = 24 atoms
Is the equation above balanced or unbalanced?
This is a balanced equation since the number of atoms of the elements is the same in the reactant and products.
Related Questions
If Mars’s atmosphere were 100% oxygen, would that make it like Earth’s?
Yes; Earth’s atmosphere is mostly oxygen.
Yes; the amount of oxygen in Earth’s atmosphere changes.
No; Earth’s atmosphere is mostly nitrogen.
No; Earth’s atmosphere is all carbon dioxide.
Answers
How do I know? I learned it in one of Film Theories videos lol
Why can more sugar be dissolved in hot water than cold water (more solute be dissolved in hot solvent then cold solvent)
Answers
Which of the following could not be an empirical formula? a CaO b CO2 c KCl d C6H12
Answers
Answer:
D. C6H12
Explanation:
An empirical formula means that the chemical formula of a compound has to be in it's simplest whole number form. In this case, D is not an empirical formula because it is able to be simplified to C3H6. All the other answers are simplified to the simplest whole number ratio. Think of it as a fraction being in simplest form.
Answer:
b CO2 is not an empirical formula.
When magnesium is added to hydrochloric acid, a gas is formed. Explain what happens in the reaction, how the gas is collected and how it could be tested to prove what it is.
Answers
Answer:
Explained below.
Explanation:
Formula when magnesium reacts with hydrochloric acid is given by;
Mg(s) + 2HCl(aq) = MgCl2(aq) + H2(g)
So, from the equation, we can see that the gas formed is hydrogen.
This hydrogen gas is collected by a method known as water displacement.
This gas can be tested for by holding a burning splint near to the top of the test container. The resulting effect should be a high pitched pop sound because the hydrogen gas will react with the oxygen gas in the air to result in a small explosion.
Given the balanced equation below, how many moles of chromium are
produced? 2Cr2O3 + 3S1 --> 4Cr + SIO3
O A. 1 mole
B. 2 moles
O C. 3 moles
O D. 4 moles
Answers
Help! Please:(
Given the following chemical equation, how many grams of water are created when 50.0 grams of oxygen react with excess hydrogen ?
2H2 + O2 - -> 2H20
Answers
Answer:
About 56.3 grams of H2O
Explanation:
What are some potential traits that are needed to become a super athlete?
Answers
Answer: Confidence, strength, potential
Explanation:
[tex] \huge \boxed{ \fcolorbox{black}{pink}{Answer}}[/tex]
20 Distinguishing Personality Traits of High-Performing Athletes
1. Self Confidence. “Self-Confidence” isn't just a phrase for cheesy motivational posters. ...
2. Strong Sense of Motivation. It takes more than a shiny medal or hefty check to motivate the world's best athletes. ...
3. Inner Desire to Succeed. ...
4. Natural Goal Setter. ...
5. Self-Discipline. ...
6. Optimism. ...
7. Sense of Belonging. ...
8. Natural Leader.
What type of mixture is Mayonnaise?
A. Homogeneous
B. Heterogeneous
C. Compound
D. Element
Answers
The molar mass of two equally sized samples of unknown gaseous compounds is shown in the table. Molar Mass Comparison Gas Molar Mass A 17 g/mol B 36.5 g/mol Which statement describes the density and diffusion of both gases at STP? Gas A has a higher density and diffuses faster than Gas B. Gas A has a higher density and diffuses slower than Gas B. Gas A has a lower density and diffuses faster than Gas B Gas A has a lower density and diffuses slower than Gas B.
Answers
Answer:
Effusion is the process of a gas being poured out through a hole diametrically smaller than the structural exit of the container.
A lighter gas effuses faster than a heavier gas.
Thus gas A has a lower density and effuses slower than Gas B.
Explanation:
The gas with a lower molar mass will have a lower density and diffuses at a slower rate. Thus gas A has a lower density than gas B and diffuses slower than gas B.
Three points should be noted here;
The density of any substance is related to the molar mass.[tex]D=M/V[/tex], where [tex]D[/tex] is the density, [tex]M[/tex] is the mass and [tex]V[/tex] is the volume of the substance.The density of the gas is directly proportional to the molar mass of the gas.Hence the gas A has lower density and the gas B has higher density as the molar mass of A is [tex]17 g/mol[/tex] and of B is [tex]36.5 g/mol[/tex].
About Diffusion please note the below points;
Diffusion is the process of movement of a substance from the area of higher concentration to the area of lower concentration.The gas with a smaller mass will diffuse slower in rate than the gas with a higher mass.Hence the gas A diffuses slower in rate than gas B.
Thus the correct answer is "Gas A has a lower density and diffuses slower than Gas B".
Learn more about the density of gas here: https://brainly.in/questions/16376906
Name the type of light interaction feeling hotter in a black shirt than a white shirt
1. Reflected
2. Absorbed
3. Transmittied
Answers
If more reactants are used in a chemical reaction, more products will be
produced. This is because *
1 point
More reactants have more atoms to react to form more products
More reactants take up the same volume
Too many products can slow down the reaction
More reactants cause the reaction to heat up
Answers
More reactants have more atoms to react to form more products.
7. A strong acid has a pH of
A) 0
B) 6
C) 7
D) 14
Answers
because acids are from PH0-PH6 and basics are from PH7-PH14
Hope this helps!
Good luck!
pls help asap!!!!
3 gasses are mixed together (N2, O2, and He). The gauge of the N2 states a pressure of 1.23 atm. The gauge on the O2 tank shows 2.3 atm. The Helium gauge is broken. When the gasses are mixed, the total pressure of the tank is 6.50 atm. What is the pressure of the He?
a) 3.47 atm
b) 2.97 atm
c) 0.435 atm
d) 10.03 atm
Answers
Answer: The pressure of the He is 2.97 atm
Explanation:
According to Dalton's law, the total pressure is the sum of individual pressures.
[tex]p_{total}=p_{N_2}+p_{O_2}+p_{He}.[/tex]
Given : [tex]p_{total}[/tex] =total pressure of gases = 6.50 atm
[tex]p_{N_2}[/tex] = partial pressure of Nitrogen = 1.23 atm
[tex]p_{O_2}[/tex] = partial pressure of oxygen = 2.3 atm
[tex]p_{He}[/tex] = partial pressure of Helium = ?
putting in the values we get:
[tex]6.50atm=1.23atm+2.3atm+p_{He}[/tex]
[tex]p_{He}=2.97atm[/tex]
The pressure of the He is 2.97 atm
Compare the seasons on Titan to the seasons on Earth.
Answers
Suppose astronomers are interested in obtaining an image of a large
area in the sky. Which radiation should astronomers observe if they
want to get images 24 hours a day, quickly and cheaply? Why?
Answers
Why the bond in water molecule os polar covalent bond, while than in chlorine molecule is pure covalent bond? Give reason?
Answers
Explanation:
The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two poles - a positive charge on the hydrogen pole (side) and a negative charge on the oxygen pole (side). We say that the water molecule is electrically polar.
To which of the three main chemical classes does Mercury (Hg) belong?
a) It's not an element in the periodic table
b) Metalloids
c) Non-metals
d) Metals
Answers
Answer:
Metals
Explanation:
It is a transition metal
The periodic table has a lot of different properties in how it is presented, one of these, is that the elements are separated in blocks by their properties.
From its position in the table, we can see that the correct option is d) metals.
The general way of knowing if an element is a metal or not, is to look if it is the D block in the periodic table.
This block contains all the transition metals (not all the metals) but every element in it is a metal. If we look for the mercury, we can see that it is in block D, just next to gold (Au).
Just from this, we can conclude that mercury is a metal, so the correct option is d.
If you want to learn more, you can read:
https://brainly.com/question/11155928
If today is Monday and it is a 1st Quarter moon, what phase will it
be on Thursday?
Answers
Answer:
Wanning gibouss 56%
Explanation:
Balance this equation. Pb(NO3)2(aq)+NaCl(aq) -> NaNO3(aq)+PbCl2(s)
Answers
Answer:
Pb(NO3)2(aq) + 2NaCl(aq) -> 2NaNO3(aq)+PbCl2(s)
Explanation:
Pb(NO3)2(aq)+NaCl(aq) -> NaNO3(aq)+PbCl2(s)
This is how it starts out.
Left:
2 NO3s1 Pb1 Na1 ClRight
1 Na1 NO31 Pb2 ClSo the place to start with this equation is to bring the Cls up to 2
Pb(NO3)2(aq)+2NaCl(aq) -> NaNO3(aq)+PbCl2(s)
But the Nas are now out of kilter.
Pb(NO3)2(aq)+ 2NaCl(aq) -> NaNO3(aq)+PbCl2(s)
Now the right has a problem. There's only 1 Na
Pb(NO3)2(aq) + 2 NaCl(aq) -> 2NaNO3(aq)+PbCl2(s)
Check it out. It looks like we are done.
What are the number of atoms in each Chemical Formula
Answers
use ideas about particles and energy transfer to explain why sweat cools you down
Answers
Answer:
So the paws in your body like ot opens up and when the wind blows on the sweat it basically cools you down
Explanation:
I hope it is what you were looking for.
What is the volume of 3.5 moles of chlorine gas at standard temperature and pressure (STP)? (3 points)
6.4 L
22 L
32L
78 L
Answers
Answer:
78
Explanation:
identify NaOH (aq) + HNO3(aq) → NaNO3(aq) + H2O(1)
Answers
Answer:
this is called acid base reaction
What are bacteria? Chapter 7 lesson 2 AYU
Answers
Answer:
a group of unicellular microorganisms
Answer:
bacteria are a type of biological cell,they constitute a large domain of prokaryotic microorganisms,single celled. The cells are all prokaryotic . This means they do not have a nucleus or any other structures which are surrounded by membranes . ... Bacteria also have small, closed-circles of DNA called plasmids present in their cytoplasm
Explanation:
100 grams of liquid iron is put in liquid water when is equilibrium reached
Answers
Answer:
when the water heats up i believe
Explanation:
b
an atom is the smallest unit of an element that retains the ___ of that element
Answers
an atom is the smallest unit of a pure substance or element that can exist and still retain the properties of the original substance or element
Sulfuric acid is an important chemical in industrial production of many products. In the “old” days, it was called oil of vitriol. It is commonly dyed brown now to alert people to its hazards because it is hugely corrosive. It is commonly found in car batteries. What is the molarity of 25.9 moles of sulfuric acid dissolved in 4.00 L of solution?
Answers
Answer:
6.475 M
Explanation:
Number of moles = 25.9 moles
Volume = 4.00 L
Molarity = ?
The relationship between the quantities is given as;
Molarity = Number of moles / Volume
Molarity = 25.9 / 4
Molarity = 6.475 M
How do human change the Earth's surface?
Human activities such as overpopulation can change the Earth's surface.
Human activities such as driving cars can change the Earth's surface.
Human activities such as mining and deforestation can change the Earth's surface.
Human activities such as poaching can change the Earth's surface.
Answers
Answer:
Human activities such as mining and deforestation can change the Earth’s surface
Explanation:
5) Calculate the volume, in liters, of 3.24 x 1022 molecules
CI2
Answers
Answer: 1.12 Liters
Explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number [tex]6.023\times 10^{23}[/tex] of particles.
To calculate the moles, we use the equation:
[tex]\text{Number of moles}=\frac{\text{Given molecules}}{\text {avogadro's number}}=\frac{3.24\times 10^{22}}{6.023\times 10^{23}}=0.05moles[/tex]
1 mol of [tex]Cl_2[/tex] occupy = 22.4 L
Thus 0.05 mol of [tex]Cl_2[/tex] will occupy = [tex]\frac{22.4}{1}\times 0.05=1.12 L[/tex]
the correct formula for the diagram is
1. 2KO
2. K2O
3. K2O
4. KOK2
Answers
Answer:
The diagram provided shows K2O, or potassium oxide.
Explanation:
Ok... so, there are two "K2O" options, so I would just choose either and hope for the best. I don't know, maybe it's a mistake...
Answer:
The answer is K2O or dipotassium Monoxide